MgCl2 is an important gradient of PCR buffer, a proven PCR enhancer- MgCl2 has many roles in a PCR that certainly lifts the reaction efficiency by many folds. Let’s find out its function and how it works.
PCR is a polymerase chain reaction, a cyclic temperature-dependent process employed for DNA amplification. The sole purpose of doing PCR is to get copies of a gene or DNA. It uses ingredients like dNTPs, primers, a template, buffer and DNA polymerase to amplify a gene.
PCR is a highly sensitive reaction and needs constant optimizations to achieve excellent results. Common optimizations are achieved by the PCR buffer, however, are not sufficient for hard templates like high GC content DNA and long-range PCR.
One among many such optimizations, the addition of MgCl2 or increasing the concentration in the reaction is a pivotal one. Research suggests that MgCl2 helps an atypical PCR reaction in many ways which eventually succeeds our assay.
But how does MgCl2 help a PCR reaction, what is the function, why it is used in the PCR and what would be the ideal concentration? Let’s find out.
I have an excellent working hand on PCR and related techniques, I have used many PCR buffers and optimized many in our lab. MgCl2 was my game changer every time and so I know about it a bit more. I will answer all of the questions related to the function of MgCl2 in the PCR in this article.
Stay tuned.
Key Topics:
What is the function of MgCl2 in the PCR?
Three important functions of the MgCl2 in a PCR reaction are,
- To improve or boost the activity of Taq DNA polymerase.
- To facilitate effective and accurately complementary primer binding.
- To increase the melting temperature (compromise the annealing temperature).
- To increase the amplification efficiency of the reaction.
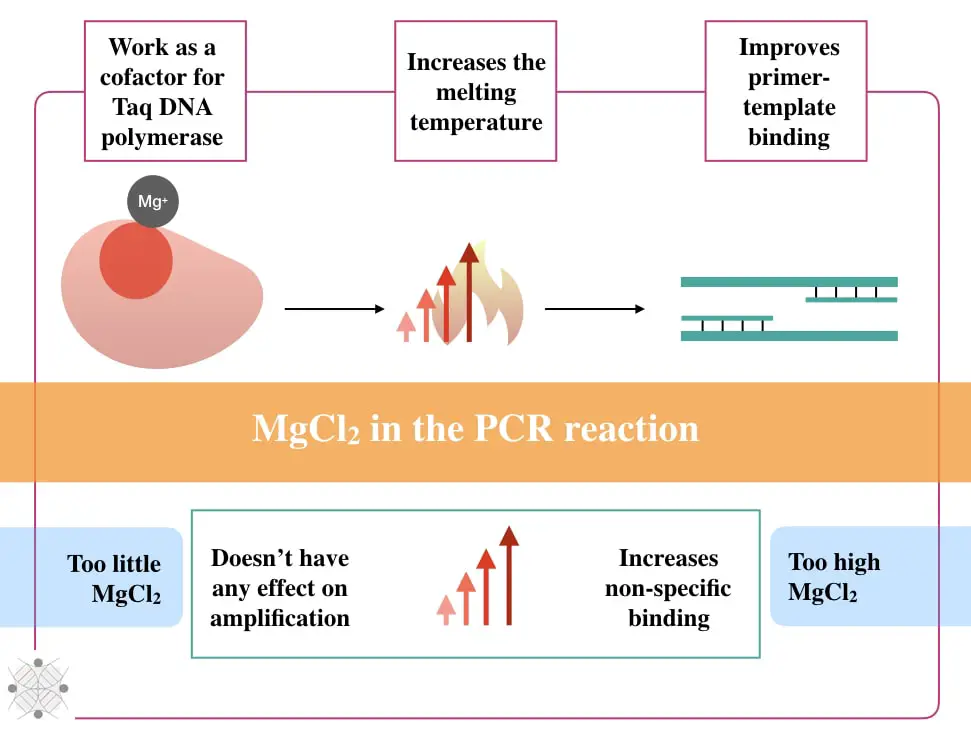
The first important function of the MgCl2 is to help the Taq DNA polymerase. Taq DNA polymerase is a thermostable DNA polymerase, that works at a higher temperature. However, like other enzymes, the Taq without a co-factor is nearly dead or can’t perform a catalytic reaction.
Co-factors are usually non-protein molecular and metal ions. The Mg2+ ions are metal ions and co-factor for the Taq DNA polymerase. In the presence of Mg2+, the Taq DNA polymerase effectively and finely synthesize the template DNA.
Again, you may wonder, How?
Research suggests that in a normal condition at 70°C temperature the Taq DNA polymerase can add >60 nucleotides per second in the presence of Mg2+ ion.
It is important to understand that two ions are utilized here. One binds to the alpha-phosphate of the template DNA while another Mg ion binds with the Asp653 and Asp830 aa of the Taq DNA polymerase active site. The collective action of both Mg+ ions greatly induces the catalytic activity of the Taq DNA polymerase.
Furthermore, keep in note that the Mg+ bind with the dNTP, works as a recognition metal ion for the enzyme to find the correct substrate. Collectively, both factors do the job for enzymes.
The second important function of the MgCl2 in the PCR is to increase the melting temperature of the reaction (Tm). The melting temperature is a temperature required to half denature a DNA template.
Increments in the melting temperature demonstrate precise primer binding. So technically it shows that the annealing temperature is compromised. The annealing temperature is a crucial factor for amplification, alteration isn’t tolerable.
However, when we compromise the annealing temperature to a small extent, it facilitates amplification of other templates as well, especially for multiplex PCR. In addition, lowering the ‘Ta’ oftentimes, gives shaper and thicker DNA bands.
The third crucial implication of the MgCl2 in the PCR is to facilitate accurate template primer binding.
As aforementioned, the Mg+ ion binds with the alpha-phosphate of a dNTP and removes the rest of the two phosphates thereby reducing the electrostatic repulsion between two DNA strands. Eventually, it stabilizes the single-stranded template DNA and prevents the renaturation of the DNA.
In such conditions, primers can effectively bind to complementary locations and complete annealing. Studies suggest that it can even facilitate the binding of primers to partially complementary sequences too.
So care must be taken!
Why do we use MgCl2 in the PCR?
All three functions viz boosting the Taq DNA polymerase action, increasing the melting temperature and reducing the reannealing capacity of the template, collectively elevates the amplification power of the reaction and increase the efficiency and yield of the PCR.
This inspires us to “must” use MgCl2 in the PCR reaction.
How to use MgCl2 in the PCR?
MgCl2 is a common constituent of any PCR buffer, but sometimes, isn’t enough! In such cases, a standard amount of MgCl2 can be added to the reaction. Use high-quality molecular grade MgCl2.
Before manual taking action, one should advise standardizing the required amount of the MgCl2 and for that, you can perform a small experiment. I have hypothetically explained it here. Six different conditions are added in the optimization module.
All six reactions would be performed using a ready-to-use PCR master mix but with a varying degree of MgCl2 concentration. Here are those six tubes.
- A standard protocol without adding a PCR buffer.
- PCR protocol with a PCR buffer
- Addition of 2mM MgCl2 in the reaction
- Addition of 3mM MgCl2 in the reaction
- Addition of 4mM MgCl2 in the reaction
- Addition of 5mM MgCl2 in the reaction
We prepared all 6 tubes with one negative control. For positive control, we added a primer that will amplify the 800bp fragment in each tube. All the tubes are run in a gradient PCR machine at the same standard annealing temperature.
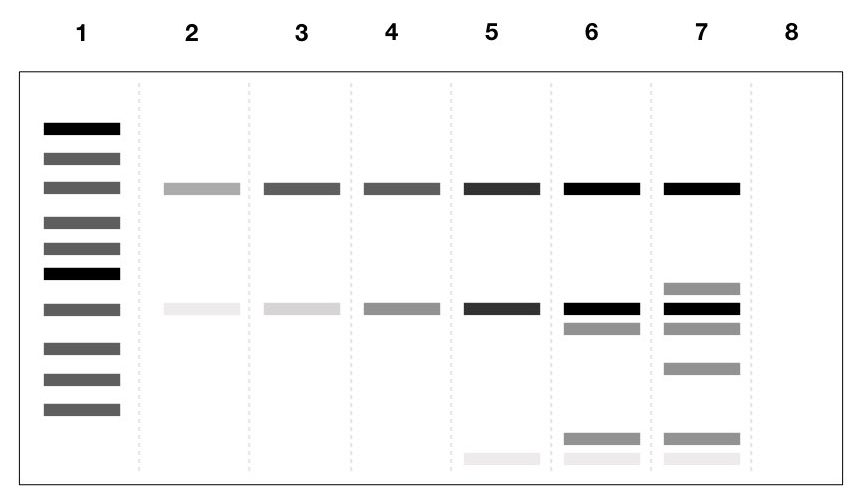
Samples are loaded into the agarose gel with a 1000bp DNA ladder and the loading pattern is as listed below,
- DNA ladder
- PCR reaction without Buffer
- PCR reaction with buffer
- PCR reaction with 2mM MgCl2
- PCR reaction with 3mM MgCl2
- PCR reaction with 4mM MgCl2
- PCR reaction with 5mM MgCl2
- Negative control
The results show that as the concentration of MgCl2 increases, the annealing capacity of the primer and the temperature are compromised and given more bands by partial complementation and primer dimer.
However, results also manifest that the reaction without a buffer isn’t a good choice to go further with. Now from this experiment, it becomes crucial to understand the effect of more and less concentration of MgCl2 in the PCR.
Too Much MgCl2: Increment in the MgCl2 concentration elevates the chances of non-specific binding, partial complementation, and primer-dimer, and also compromises the annealing temperature.
Put simply, you will get many bands in a gel. Try it, the results would not be even conclusive.
In some critical reactions, where the DNA polymerase proofreading is also included, adding too much MgCl2 will cause an error during replication by promoting wrong nucleotide insertions.
Too little MgCl2: An inadequate amount of MgCl2 isn’t of any use. It doesn’t have any direct effect on the reaction, it can’t perform the function for what, it has been added. It eventually fails the reaction or gives the same results as the previous one.
In which conditions or for which reaction do we need MgCl2?
Now, this is important. When do we need to add some extra amount of MgCl2? See, Ideally, for conventional PCR amplification with standard conditions, the part present in the buffer is enough. No need to do more, unless the results are satisfactory. However to achieve excellent PCR amplification modifications are required.
Higher GC content templates are certainly hard to amplify, with a normal protocol. It needs a special treatment to effectively amplify it. Adding MgCl2 to such a reaction will help achieve success.
Again, for higher GC templates it reduces the annealing temperature, prevents rapid renaturation and facilitates accurate primer binding.
In one more scenario with a longer template MgCl2 change the game. Longer templates are also hard to amplify completely, for example, a fragment of 2Kb or 5Kb. Though a special setup, protocol and temperature conditions are required, adding a pinch of MgCl2, one more time, helps achieve amplification.
What is the ideal concentration of MgCl2 in the PCR?
One standard PCR buffer comes with 1.5 to 2mM MgCl2. However, one can use MgCl2 between 2 mM to 5 mM if required. More or less than this (for optimization purposes) can’t work.
Remember higher the concentration of MgCl2, the more the chances of non-specific amplification.
In my opinion, 2.5 mM additional MgCl2 is sufficient in the reaction, even for, multiplex PCR too, unless you are doing something out of the box. In one scenario I remember when we were working on standardizing the thalassemia mutations (codon 51/52), we get amplification after adding 5.5 mM MgCl2.
Although the quality of results wasn’t that good, it worked.
Wrapping up:
“Less is more.”
Molecular genetics is all about playing with different components and choosing the right combination.
I always believe in not optimizing things unnecessarily. Try to achieve precision with less quantity of ingredients. Practice using and optimizing with less quantity. Trust me, this will surely make you a better scientist in the future.
MgCl2, now you understand, is a crucial ingredient in PCR as well as in DNA extraction too. Adding some extra will surely increase amplification power, but you have to choose how much you want.
I think this article will perhaps help you in your genetic endeavor.
Sources:
Miller Bill R, Beese Lorena S, Parish Carol A, Wu Eugene Y. The Closing Mechanism of DNA Polymerase I at Atomic Resolution. Structure. 2015 Sep;23(9):1609–20.

What do you think about MgCl2 in solution prior to PCR? Would it prevent Taq polymerase from binding by merging DNA strands together?
what if we get band in negative control of pcr as well ? what will be the possible reason ?
Very helpful article dear. Keep your good work
Thank you Poonam Ray for appriciating our work.
This is informative article for whom to starting up their PCR for dounstream apllication.