“VDJ recombination is a crucial process to produce various types of antibodies against antigens. Explore the structure of an antibody and learn about the concept of VDJ recombination.”
Antibody diversity has a significant role in producing different combinations of antibodies as per the requirement. And this whole process– of producing different combinations is backed by a genetic mechanism, known as VDJ recombination.
VDJ are Variable, Diversity and joining segments of DNA that transcribe into various polypeptides by producing different combinations. In this article, I will explain to you the structure of the antibody, the mechanism of VDJ recombination and the concept of antibody diversity.
The present article will help you understand how immunological components will work effectively and how genetic mechanisms are involved in the process. This article will be published as part of 3 articles.
So this is the first article of this series.
Stay tuned.
Key Topics:
Structure of Antibody:
First, let’s understand the structure of antibodies.
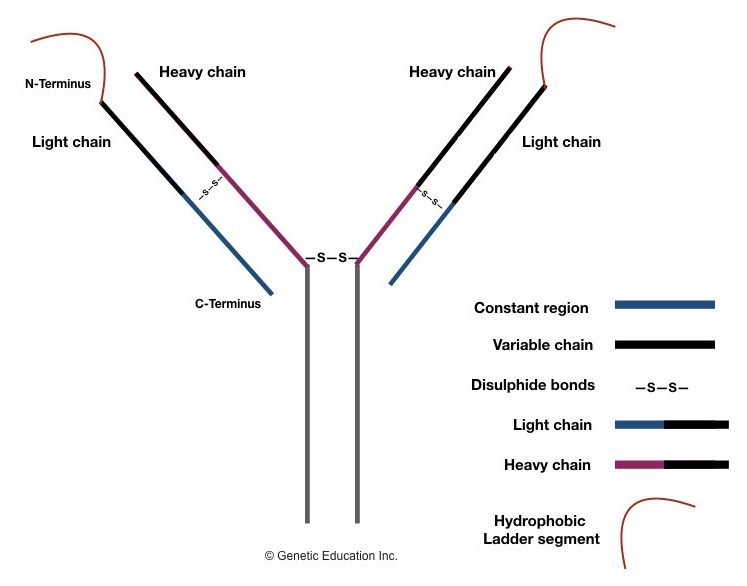
Structurally, an antibody is a Y-shaped protein molecule that contains two identical light chains and two heavy chains. Each light chain is made up of 220 amino acids, and each heavy chain is made up of about 440-450 amino acids and these chains are allied by disulfide bonds.
A variable region present on each heavy and light chain at the N terminus end makes it antigen-specific. A constant region present on both chains is non-variable, coated on the C-terminus end and supports the structure of antibodies.
A constant region constructs an effector functional domain, which is responsible for the interaction of that antibody with other immune components. Depending upon the functionality of the antibody, it is characterized into 5 major classes: IgM, IgD, IgG, IgE and IgA.
IgM and IgD are referred to as surface antibodies and are embedded into the cell membrane. Every antigen initially comes in contact with these surface antibodies.
Depending upon the function and location, two types of antibodies are present: The membrane-bound antibodies, present on the membrane of the cell and the second is secreted antibodies, secreted from the B lymphocyte cells, induced by the antigen attack.
The light chain of the antibody is of two types: kappa light chain and lambda light chain and is determined by the constant region of a light chain. The constant region plays an important role in light chain synthesis, irrespective of that it remains unchanged.
The arrangement of the antibody is a bit complex but if you read it over and over you will understand. Please examine the image carefully to get more clarity. Now, it’s crucial to know that various antibody light chain coding gene sequences are located on different chromosomes.
Indeed we can’t say it’s a ‘gene,’ those are gene segments, actually. Because the segmental rearrangements prepare various polypeptide chains for variable regions. Thus, various heavy and light chains, a constant region, lambda and kappa chains etc. are synthesized by the combination of various gene segments.
Let’s first understand the arrangement of gene segments.
Arrangements of gene segments:
Light chain
The light chain is of two types: kappa light chain or lambda light chain.
Let’s denote the variable region as V (the variable regions are actually coding exons that make different polypeptides), a constant region as C and Joining segments as J (joining segments are just non-coding intervening sequences).
Keep in note that each chain (whether it is a light or a heavy chain) is made up of a long chain of polypeptides with an N terminus and C terminus, it starts with the N terminus at the variable region and ends at C- terminus towards the constant region. See the figure to understand it properly.
A number of gene segments for the different types of polypeptide chains:
| Kappa Gene Segments | Lambda gene segments | Heavy Chain | |
| Varibale region | Vk = 38 | Vƛ = 33 | VH = 38 to 46 |
| Joining region | Jk = 5 | Jƛ = 5 | JH = 6 |
| Diversity region | 0 | DH = 23 | |
| Constant region | Ck + 1 | Cƛ = 4 | CH = 9 |
Kappa light chain:
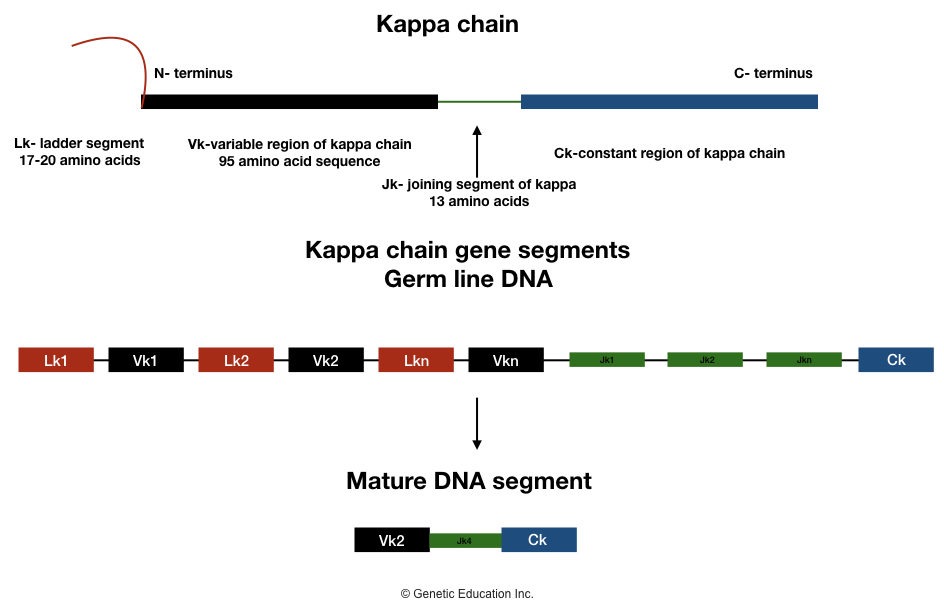
At the N terminus end, the Vk gene segment codes for 95 amino acids, Three Jk gene segments code for 13 amino acids, and at the C terminus end, the Ck gene segment codes for the remaining amino acid of a light chain.
One chain of hydrophobic leader sequences is also present at the N terminus end of the kappa light chain which helps in the transportation of antibodies at the site of action. It is made up of 17-20 amino acids.
Various antigen-specific antibodies are synthesized by the event of recombination of different Lk-Vk and Vk-Jk segments during the development of B lymphocytes. The entire segment of Lk-(Vk)Jk-non coding sequence-Ck is transcribed.
Now at the end, all non-coding parts of the gene segment are removed from the mature kappa chain. Only Vk-Jk-Ck sequences will remain intact and participate in the development of a particular type of antibody.
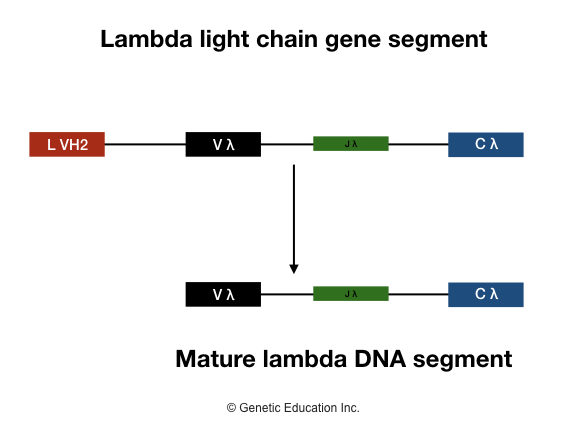
Lambda light chain
These gene segments are assembled during B lymphocyte maturation as well and create different antibodies through the recombination of different gene segments. The maturation process will remain as kappa chain gene segments. Here the segments are denoted as Jλ, Vλ, Cλ.
Heavy chain
The genetic information for coding heavy chain gene segments is arranged on LH-VH, JH and CH gene segments. Here the kappa or lambda variable region will remain the same as a light chain, nevertheless, one additional gene segment is present in the heavy chain segment, which is a “diversity segment” or “D segment”.
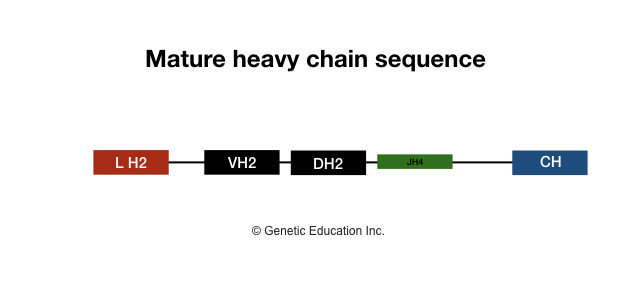
D segment awards additional diversity to the antibody which is present in the variable region of a heavy chain. The D segment is made up of the 2-13 amino acid long polypeptide chain. Here 4 separate CH gene segments are present for each Ig class heavy chain.
In humans, a total of 9 to 10 functional CH gene segments are present. The list of different segments is given in the table. In CH gene segments of the heavy chain, the intervening sequences are typically pseudogenes (sequences which are similar to the functional gene but are non-function), during the maturation of B lymphocyte pseudogenes are removed.
Related articles:
- What are pseudogenes and How Do They Differ From Genes?
- How Different Types of Pseudogenes are Formed?
- The Role of Gulo Pseudogene.
Depending upon the function and location, two types of antibodies are present: The membrane-bound antibodies, present on the membrane of the cell and secreted antibodies, secreted from the B lymphocyte cell, induced by the antigen attack.
Now the membrane-bound antibodies are fixed on the cell membrane and embedded into the lipid layer of the membrane. The C terminus end of a heavy chain antibody is hydrophobic in nature which helps them to fix in the lipid layer, whereas the C terminus end of circulating antibodies has the hydrophilic end which makes them circulate freely.
IgM and IgD are fixed antibodies present on the surface of the cell, others are freely circulating in the circulatory system and depending upon the type of antigen, the specific antibody is secreted.
D(V)J recombination process:
Susumu Tonegawa won the Nobel Prize for finding the principle of generation of antibody diversity in 1987.
In the process of recombination, first, the recombinase enzyme came into the picture that recognizes the RSS sequences- Recombination Signal Sequences. Interestingly RSS are located adjacent to each gene segment, viz near to Variable, Joining and diversity segments.
Recombination Signal Sequences are very crucial for in vivo recombination as well as for in vitro studies. Recombination between varied gene segments only occurs when the RSS has a varied length of a spacer sequence.
The spacer sequences are present between the heptane and nonamer elements of RSS. The heptamer and nonamer sequences are nearly conserved.
Different types of recombinase are RAG1- recombination activating gene 1, RAG2- recombination activating gene 2, Artemis nuclease, TdT- terminal deoxynucleotidyl transferase, etc. And are involved in the NHEJ DNA repair pathway.
Other enzymes besides these are DNA protein kinase, DNA ligase IV, non-homologous end-joining factor 1 and X-ray repair cross-complementing protein 4. All perform different functions in the whole process.
For example, ligase IV joins or ligates the final recombined fragment to construct a receptor.
The RAG1 protein initiates the process by recruiting the recombinase to the site of recombination where it creates a single-strand nick between the first base of RSS and coding segment DNA sequence.
At this recombination center, a hairpin loop at the coding segment occurs due to free 3’ and 5’ ends on the same strand. The hairpin formation is crucial to manufacturing different combinations.
The rest are the signal sequences- non-coding ones, ligated and form circular DNA which is later incorporated into the genomic DNA. Other proteins mentioned above also function in a sequential complicated process to ligate a varied gene segment of V(D)J.
In the last step, the exonuclease removes some non-useful nucleotides and DNA polymerase inserts nucleotides to make segments compatible for rejoining. At the end of the process, a highly variable antigen-binding region is formed to invading pathogens.
Following this pattern, millions to billions of various, novel and known antigen-binding receptors are synthesized every single day. However, in order to produce a specific receptor or antigen-binding segment, the DNA sequences explained above must be in a sequential manner to form the correct chain of amino acids. If not, the cell will die soon, because an abrupt combination is no longer needed.
So this is a broad overview of the VDJ recombination process, we know some enzymes and pathways but a large pool of information is still unknown to us.
Generation of antibody diversity by class switching:
IgM and IgD are called primary antibodies because both are present on the surface of the cell membrane and primarily interact with antigens. The secondary antibodies IgE, IgA and IgG are secreted by B cells depending upon the antigen present on the cell membrane hence this class of antibodies is termed secondary antibodies.
Depending upon the type of antigen, instead of making one class of antibody, the mature B cell starts making another class of antibody, this phenomenon is called antibody class switching.
Once the primary class of antibodies recognizes the antigen, the B cell differentiation is initiated depending upon the type of antigen present on the cell membrane. Even though it synthesizes some specific antibodies, here it synthesizes antigen-specific antibodies.
The antibody Constant segment decides which class of antibody will produce the immune response, here as the primary antibody is IgM, the CH gene segment is preliminarily the same for all antibodies.
In class switching, instead of producing IgM antibodies, some mature B lymphocytes produce another class of antibody based on the gene segment arrangement of CH.
This antigen-induced secondary antibody production is called class switching. The mechanism is governed by the rearrangement of different DNA segments of V, D, J and C regions followed by class-switching recombination.
Importance of V(D)J recombination:
Our body faces many brutal attacks by viruses, phases, bacteria, fungi and other chemical particles, every day. A body needs to invade through different mechanisms.
Foreign particles broadly categorized into “antigens” initially come in contact with Antigen antigen-presenting cells. Antigens are modified and synthesized as per the requirements.
To invade many attacks, different gene segments of the heavy chain, light chain, constant and joining region combine by the process of recombination and create different combinations.
Nonetheless, some combinations can’t work, whilst memories for some common combinations are saved for future use. This means that when the same pathogen or foreign antigen attacks, cells immediately synthesize immunoglobulins by using the saved memory.
But On the downside, chromosomal DNA portions, especially those involved in antibody diversity, break and rejoin many times, in fact, thousands to millions of times during the lifetime of antibody receptor synthesis. During this process, some fragments can be repaired and some remain unrepaired and resulting in serious genetic problems.
Interesting articles:
Wrapping up:
VDJ recombination and segmental antibody generation is a complex process, however, can help us understand the antibody generation process and thereby produce new approaches to invade pathogens.
Each fragment or segment should be synthesized correctly, otherwise, it will lack the functionality for what it would involve in the antibody generation process. I hope this article makes sense for you.