“Post-translational modifications, abbreviated s PTMs are the biological modifications that occur after protein synthesis which alters protein structure and function. Common PTMs are phosphorylation, Methylation, acetylation, lipidation, hydroxylation and ubiquitination. “
- Post-translational modifications occur following protein synthesis.
- It’s a chemical/enzymatic catalytic reaction to modify different proteins for different purposes.
- It usually happens at the C or N termini of amino acids.
- 400 different types of PTMs are reported.
- PTMs provide functional diversity to proteome.
Replication, transcription and translation are biological pathways very essential and important for life on earth. It’s a process, an enzymatic catalytic reaction forms a chain of amino acids- a protein from the DNA.
The intermediate molecule, the mRNA, transfers the message to make a correct protein involving varieties of molecules and pathways.
Replication makes a copy of DNA, transcription forms the mRNA from the DNA and translation makes a chain of amino acids using the message encoded into the mRNA.
Replication and transcription occur in the cell nucleus while the translation occurs at the ribosome in the cytoplasm. After completion of protein synthesis (translation), the amino acid chain undergoes post-translational modification to enfold more in secondary, tertiary and quaternary structures and make different proteins.
So the PTMs are as important as the threes’ of the central dogma; if it doesn’t occur, a proper and required protein can’t form.
I have read many articles on the present topic, but some are too basic and some too complicated. So to serve better and understandable content, I am trying to write this article on post-translational modification.
In the present article, I will explain the process, example and types of post-translational modifications and related topics. I hope you will like it.
Overview of the PTM processes:
| Phosphorylation | Addition of phosphate group to the amino acid of the protein. |
| Glycosylation | Manufacturing glycoprotein by incorporating sugar or carbohydrate moiety to protein. |
| Ubiquitination | Linking a ubiquitin protein to another ubiquitin or to other proteins. |
| acetylation | Addition of acetyl group to the lysine amino acid of the protein. |
| Hydroxylation | Addition of hydroxyl group to the amino acid |
| Deamination | Amino acid modifications convert one form of amino acid to another. |
| Prenylation | A type of lipidation adds fatty acid to the amino acid |
| Proteolysis | The process of protein degradation |
Key Topics:
What are post-translational modifications?
We need to understand some points to clarify the PTMs, we are starting from the genetic code. A triplet mRNA code converts into individual amino acids. Each amino acid has one functional group and one hydroxyl group on each end.
PTMs form various functional proteins by modifying 20 different amino acids. The triplet code is known as the genetic code. The post-translational modifications are abbreviated as PTMs or PTM of proteins. A few common PTMs are enlisted here,
- Removing the methionine- AUG code from the mature chain.
- Adding a functional group to the amino acid or polypeptide chain through phosphorylation, glycosylation, ubiquitination and methylation etc.
- Forming disulfide bonds between cysteine residues
- Changes the function
Importantly, PTMs are reversible or irreversible chemical changes of post-protein synthesis mediated by different enzymes of protein biosynthesis. We can say, a protein may reprocess to form another type of or the same type of protein having differences in functionality.
Here, reversible means that it can revert back to its original structure or original protein can be reformed, if needed.
PTMs befall following the translation but not immediately, meaning, some occur immediately while some at later stages of protein folding. We can divide the life of a protein in three-stage: manufacturing, processing and folding.
- Protein manufacturing- Constructing a protein using raw material in the process of transcription and translation.
- Protein processing- Meaning post-translational modifications.
- Protein folding- forming different forms of protein- secondary, tertiary and quaternary.
So as we said, several PTMs occur immediately, after which, it affects the efficiency, tendency and conformational stability of the protein, indicating, it influences the folding process directly. Thereby promptly influences the structure and function of a protein.
Notedly, other PTMs occur even after protein folding or localizing into the cell.
Instead of abrupting the structure, it only alters the biological activity and functionality of the protein.
Broadly, genome and proteome are the two most commonly studied ‘omics’, consisting of studying genes/DNA and protein, respectively. The Genome is a complex thing! But let me tell you, the proteome is even more complex, comprising thousands of modifications and processing pathways to diversify protein functionally.
The human genome consists of 25,000 known genes but the proteome consists of more than 1 million protein forms, studies show. So we can say, studying proteome is much more complex.
This can be even more understandable when we look into the number of amino acids manufactured. A single gene is capable of forming many different mRNA transcripts and henceforth many proteins are certainly formed.
PMT is one of the factors that diversify proteins, besides, processes such as alternative splicing, alternative promoters, differential transcription and mRNA splicing. Keep in mind that proteome complexity diversifies protein in various ways.
The clear objective of PTMs is to regulate cellular metabolism and activities, to make us survive, consequently. We can say, as a gene diversifies positively, it makes new proteins for us.
Coming back to the PTMs, now let see several different types of PTMs.
Types of Post-translational modifications (PTMs):
Several examples of post-translational modifications are;
- Phosphorylation
- Methylation
- Glycosylation
- Ubiquitination
- N-acetylation
- S-nitrosylation
- Proteolysis
- Lipidalation
- SUMOylation
- Palmitoylation
- Sulfation
- Myristoylation
- Prenylation
- Alkylation
- Glycylation
- Biotinylation
- Glutamylation
- C-terminal amidation
- Selenation
- Lipoylation
- Isoprenylation
- Phosphopantetheinyl Nation
- Acetylation
- AMPylation
- Proteolysis
- Deamidation
- Hydroxylation
- Neddylation
- Prenylation
- Nitrosylation
Definition:
The enzymatic reaction that occurs following the protein synthesis to make various functional protein products is known as post-translational modifications or PTMs.
Mechanism of action:
Amino acids link to make a polypeptide chain through peptide bonds. Structurally, the amino acid has one amino group on one side and a carboxyl group on another side.
It also has the “R”- functional group, which is anything such as other amino acids, lipid, phosphate or sulfur groups, etc. This “R”- functional group is the site for PTMs.
Usually, post-transcriptional modification initiates by disrupting the peptide bonds between amino acids in most cases. Next, depending upon the kind of PTM, the enzyme initiates its catalytic reaction.
For example, to add a phosphate group, enzyme phosphatase mediates catalytic reaction in the phosphorylation process. The process occurs immediately after translation or later on after protein folding.
10 most commonly studied PTMs example:
Methylation
- Type: reversible PTMs
- Enzyme: methyltransferase
- Target residues: Arginine and lysine
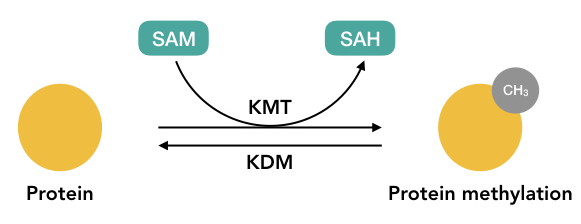
Methylation is one of the most common post-translational modifications in which methyltransferase inserts one methyl group into an amino acid. Methylation is both an irreversible or reversible type of PTM, addition and removal of methyl groups occur simultaneously when required.
SAM- S-adenosyl-l- methionine is the primary methyl group donor not only in protein methylation but also in DNA methylation. Usually, arginine and lysine are the most common target for methylation.
Two common methyltransferases are PRMTs and HKMTs acronyms as protein arginine methyltransferases and histone lysine methyltransferases, respectively. Research data also shows that arginine can only methylate 1 or 2 times but lysine can methylate 03 times.
When the methyl group adds to the nitrogen (N-methylation), the process occurs irreversibly but when the methyl group adds to the oxygen (O-methylation) the process occurs reversibly.
The best example to explain is the methylation and demethylation of histones. Histones of proteins are linked with the DNA and help DNA to package on chromosomes via nucleosome assembly.
The addition and removal of the methyl group certainly affect gene expression. It regulates the number of ‘transcripts’ forms during transcription. It helps in epigenetic regulation.
Some of the advantages of protein methylation are,
- Regulation of gene expression
- Provides protein stability
- Cellular processing
- Protein-protein interactions
- Heterochromatin assembly
- Protein modification events
- Subcellular localization of proteins
- Provides promoter binding affinity
- Epigenetic silencing.
Notedly, eukaryotes have methylated arginine seen in both histones and several non-histone proteins. Cancer, Angelman syndrome and lipofuscinosis occur by defective methylation process, recent studies suggest.
Methylation errors are linked with any other types of genetic diseases as well.
Phosphorylation
- Type: reversible PTMs
- Enzymes: Kinase and phosphatase
- Target residues: Threonine, tyrosine, serine and histidine
Yet another most studied PTM is the phosphorylation of protein and is commonly noticed in both prokaryotes and eukaryotes. Phoebus Levene reported the process of phosphorylation for the first time in protein vitellin in 1906.
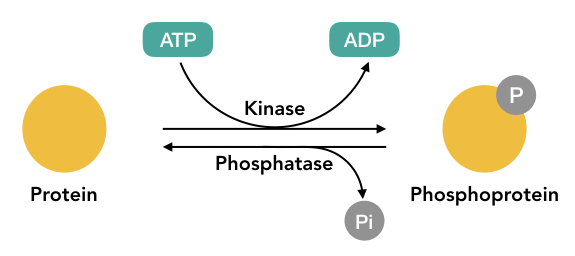
Two enzymes kinase and phosphatase catalyze the entire process of phosphorylation and dephosphorylation, respectively.
Adenosine triphosphate- ATP is the phosphate donor for the process to occur. Interestingly, it alters protein function for a short time, meaning, protein function can be reverted when dephosphorylated.
Protein phosphorylation is a kind of reversible PTM, adds and removes phosphate from amino acids especially, threonine, tyrosine and serines.
The important consequence of phosphorylation is that it adds a negative charge to amino acids and makes a protein more electronegative. Charge modification changes the nature of protein from hydrophobic to hydrophilic.
Phosphorylated (negatively charged) proteins can interact with other positively charged proteins activating many cellular processes.
Phosphorylation is applicable in,
- Cell cycle regulation- growth and apoptosis
- Signal transduction pathways
- Activation and deactivation of enzymes
- Activation and deactivation of protein receptors
- Activation of cell membrane channels
Glycosylation:
- Type: irreversible
- Enzymes: broadly categorized into glycosidases
- Target residues: Asparagine, threonine and serine.
Much like phosphorylation, glycosylation is also a common and well-studied PTM, however, is a bit complex and complicated process. Glycosylation adds carbohydrate moiety to the amino acid of a particular protein, thereby forms a glycoprotein.
Studies show that approximately 50% of our proteomes are various glycoproteins. Different catalytic enzymes work depending upon which type of sugar will be added.
It’s a type of irreversible PTM, enzymes involved in the whole process are categorized as ‘glycosidases’. Common two glycosylation types are N-linked and O-linked, besides C-glycosylation and phospho-glycosylation also occur.
The N-linked glycosylation occurs at the amide nitrogen of the N-termini of amino acid while the O-linked occurs at the oxygen atom. The N-linked and O-linked glycosylation occurs on serine and threonine, respectively.
Importantly, glycosylation has a major role in protein structure folding, conformation, stability, distribution and activity.
Immunoglobulins i.e, antibodies are the best example of glycosylated protein in which attachment of sugar moiety to protein forms different immunoglobulins.
Several applications of glycosylation are;
- In adaptive immunity
- Determining blood groups
- Provides structural support to membrane
Ubiquitination:
- Type: reversible
- Enzymes: E1, E2 and E3
- Target residues: commonly lysine but occur on all 20 amino acids.
A type or reversible PTMs- ubiquitination was first reported by Gideon Goldstein in 1975.
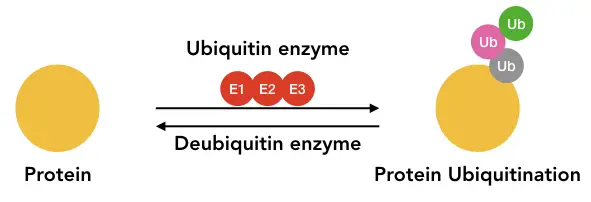
Ubiquitin is a protein, governs the catalytic reaction of ubiquitination that works in regulating and degrading protein. It’s an 8kDA size small protein that binds to the substrate ubiquitin-protein residue.
Enzymes E1, E2 and E3 govern the whole process. Usually, the reaction occurs by forming the isopeptide bond between ubiquitin and substrate protein at lysine, threonine, cysteine and serine residues. However, it may occur on all 20 amino acids. The bond links the C terminal of the ubiquitin and N-terminal of the target protein.
Research also shows that a single or many ubiquitins may attach to the target protein, known as monoubiquitination and polyubiquitination.
One of the important properties this reaction has is that the ubiquitinated substrate may further go into phosphorylation and acetylation. The enzyme deubiquitinase removes ubiquitin from the target substrate.
The process is mainly involved in the degradation of protein, DNA repair signaling, inflammatory response and organelle biogenesis. Besides, It has a pivotal role in other pathways and reactions; some of these are;
- Regulation of transcription, replication and DNA repair,
- Proliferation, signal transduction and apoptosis
- Innate immune signaling
- Intercellular trafficking and virus budding.
Error in the ubiquitination pathway causes inflammatory disorders, metabolic syndromes, type 2 diabetes and even cancer.
N-acetylation
- Type: reversible and irreversible
- Enzymes: lysine acetyltransferase
- Target amino acid: lysine
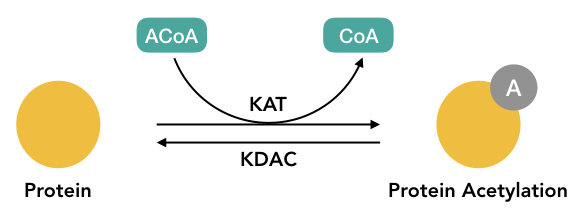
In 1964, Allfrey VG had reported the first acetylation modification in isolated calf thymus nuclei. Two enzymes KAT and HAT have a definite role in the process are lysine acetyltransferase (KAT) and histone acetyltransferase (HAT).
The N-acetylation process follows both reversible and irreversible mechanisms of PTMs, transferring the acetyl group to the nitrogen residue of the target protein. Interestingly, all eukaryotes have N-acetylation commonly reported.
The process of chromosome relaxation and condensation is highly relying on acetylation. The lysine amino acid of the histone protein- helps in chromosome packaging, plays a vital role here.
Thereby it indirectly governs the process of transcription by acetylation and deacetylation. Lys, Ala, Cys, Gly, Met, Pro, Val, Ser and Arg are common target amino acids for acetylation.
When N-acetylation occurs at the lysine residue of the histones, It relaxes chromosome condensation and promotes transcription. On the other side, deacetylation results in chromosome condensation and blocks transcription by disallowing DNA to transcription.
HAT- acetyltransferase and HDAC-histone deacetylase co-repressor catalyze both reactions, respectively. Importantly the process known as hyperacetylation maintains genomic integrity by gene silencing.
The HAT uses the acetyl-CoA as a cofactor to insert the acetyl group while the HDAC removes the acetyl group from the lysine. Three common types of acetylation are N(alpha)- acetylation, N(epsilon)- acetylation and o-acetylation.
This PTM has a major role in genome and chromatin stability, regulating transcription, regulation of cell cycle, nuclear transport, protein-protein interaction and actin nucleation.
Rapid aging, Huntington’s disease, Parkinson’s disease, immune disorders, cardiovascular problems and cancer are common diseases associated with dysregulation of acetylation.
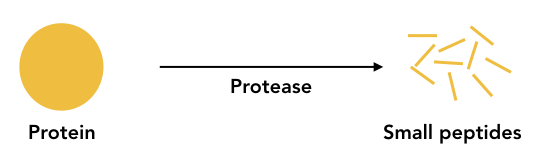
Proteolysis
Proteolysis is a process of protein degradation that occurs via the catalytic reaction of protease. It performs a vast array of biological functions. All eukaryotes have the enzyme protease present; which is the largest group enzyme in humans. Post-translational modifications such as cleaving polyproteins, degrading precursors, signal sequence removal, Removal of N-terminal methionine and other protein degradation activities occur by proteolysis.
Palmitoylation:
- Type: not known
- Enzyme: palmitoyl transferase
- Target amino acids: glycine, cysteine, serine, lysine or Threonine
It’s a kind of lipidation or a type of lipid modification that uses palmitoyl transferases- PATs enzyme for PTMs. It was first reported by Bartels D in 1999.
S-palmitoylation process is the best example of present PTM which regulates membrane localization (like an On/Off switch).
Enzyme PAT adds the Palmitoyl group of the palmitoyl-CoA to the cysteine amino acid of the protein. It serves, a permanent anchoring of palmitoylated protein to the membrane.
Put simply, Lipidation occurs when a fatty acid attaches to the protein. Here, enzyme PAT governs the covalent binding of fatty acid- Palmitate with the residue of protein such as glycine, cysteine, serine, lysine or Threonine.
Enzyme acyl protein thioesterases govern the depalmitoylation process by removing the fatty acid from the protein.
The process has functions in protein regulation, signal transduction, protein interaction, neuronal development and apoptosis. Neurological problems and cancer are common diseases caused by the dysregulation of Palmitoylation.
Prenylation
- Type: Irreversible
- Enzyme: Farnesyltransferase
Yet another type of lipidation is prenylation reported by Yuji K et al. It’s an irreversible PTM. 2% of all human protein is prenylated; all proteins of the Ras superfamily are mainly prenylated.
Enzyme farnesyl transferase (FT) governs the catalytic reaction of adding the farnesyl or geranylgeranyl group to the cysteine residue of the protein.
The present PTM has a significant role in various biological activities and protein regulations such as cell growth, endocytosis, protein-protein interaction and trafficking of proteins.
Abruption may cause neurodegenerative, bone, metabolic and cardiovascular disorders.
Hydroxylation:
- Type: irreversible
- Enyzme: Hydroxylase
Hydroxylation inserts the hydroxyl group at the amino acid of a protein. The enzyme hydroxylase governs the process that helps to convert hydrophobic compounds into hydrophilic.
It’s the most common post-transcriptional modification that adds the functional group to protein thereby regulates protein-protein interaction. It’s an irreversible process.
Deamination:
The process of deamination is classified under amino acid modifications which is a conversion of amino acids from one to another form. For instance, Asparagine to aspartic acid and Glutamine to glutamic acid or polyglutamic acid.
Protein PTMs and role in disease development:
Abnormal PMT changes result in disease. Let me summarize things! When the post-translational modifications can’t occur precisely it causes health problems. Those are,
- More unwanted PTMs,
- Lower PTMs,
- Hyper or hypoactivity of the enzyme involved.
- Abnormal pathway of PTM
- Off-target PTM
Neurological disease and cancer are the two most common problems that occur by abnormal PTMs. Several associated diseases are Huntington’s disease, Parkinson’s disease, Alzinemr’s disease and different types of cancer.
It alters or influences the biological process of protein-protein interaction, cell metabolism, cell differentiation, cell division process, DNA repair, protein folding and protein DNA interaction.
Summary:
| PTM | Enzyme | Target amino acid | Type |
| Methylation | Methyltransferase | Arginine and lysine | Reversible |
| Phosphorylation | Kinase and Phosphatase | Threonine, tyrosine, serine and histidine | Reversible |
| Glycosylation | Glycosidases | Asparagine, threonine and serine | Irreversible |
| Ubiquitination | E1, E2 and E3 | Commonly lysine but all 20 amino acids | Reversible |
| N-acetylation | Lysine acetyltransferase | Lysine | Reversible and irreversible |
| Palmitoylation | Palmitoyl transferase | Glycine, cysteine, serine, lysine and threonine | Not known |
| Prenylation | Farnesyltransferase | Not specific | Irreversible |
| Hydroxylation | Hydroxylase | Not specific | Irreversible |
Wrapping up:
The post-translational modifications have a huge list, in short, we can understand that any change in protein structure (after translation) that changes the structure, function and how a protein behaves are considered as PTMs.
The addition or removal of functional groups, molecules and other amino acids change protein functionality and structure.
Notwithstanding, some modifications are useful and happen intentionally while some are not. Unwanted PTMs cause serious health problems and genetic disorders.
Sources:
Aebersold R, Agar JN, Amster IJ, et al. How many human proteoforms are there?. Nat Chem Biol. 2018;14(3):206-214. doi:10.1038/nchembio.2576
Shahin Ramazi, Javad Zahiri, Post-translational modifications in proteins: resources, tools and prediction methods, Database, Volume 2021.