“The role of alcohol in DNA extraction is to precipitate, wash and store DNA. Let’s find out how it executes each function.”
Liquid-liquid DNA extraction highly relies on various chemical combinations and solutions. Lysis buffer, an utmost requirement of DNA extraction, is needed to lyse cells. The buffer here contains many ingredients that help in the extraction process.
In the next step, the liquid nucleic acid extract is precipitated and eluted and stored. The classic Maniatis and Sambrook protocol uses this principle to extract DNA. Here alcohol performs crucial functions.
Precipitates of DNA are visible, the cotton-treat-like structure appears after alcohol treatment. But how does it occur? In addition, how other two functions are executed and what is the type of alcohol used for precipitation, washing and storing DNA?
This article is a practical guide and a part of DNA extraction. We will understand the role of alcohols- isopropanol, ethanol and methanol in DNA extraction.
“Reaction between solute and solvent creates an insoluble solid substance, called a precipitate and the process is referred to as precipitation.”
Stay tuned.
Key Topics:
What is the role of alcohol in DNA extraction?
Alcohol performs three different functions in DNA extraction, which are precipitation washing and storing.
DNA precipitation:
To better understand the present mechanism, let’s start with some basics and background information. Two phenomena of polarity and dielectric constant are important to discuss here.
Polar and nonpolar molecules dissolve in polar and nonpolar solutions, respectively. Solute and solvents dissolve properly only if they have a similar structure. DNA is a polar molecule and soluble in water as water is also partially polar.
Chemically water has a partial negative charge near the oxygen atom and a partial positive charge near the hydrogen atom. The negatively charged phosphate backbone of DNA interacts with the positive charge of water.
This interaction dissolves DNA in water and stabilizes its structure. Technically it’s the electrostatic interaction between the H+ of water and PO3– of the DNA.
In addition to this, DNA is also a hydrophilic molecule (Hydro means water and philic means to attract) which literally means, it attracts water and solubilizes. But that’s not the case with alcohol.
When we add alcohol and salt, DNA precipitates into a visible form. So what exactly happens there? Now precipitation can be explained by the dielectric constant of water and alcohol.
According to Coulomb’s law, “force of attraction between two opposite charges is inversely proportional to the dielectric constant.” The dielectric constant of water is ~80 and alcohol is 24.
As per the law, alcohol repeals DNA stability.
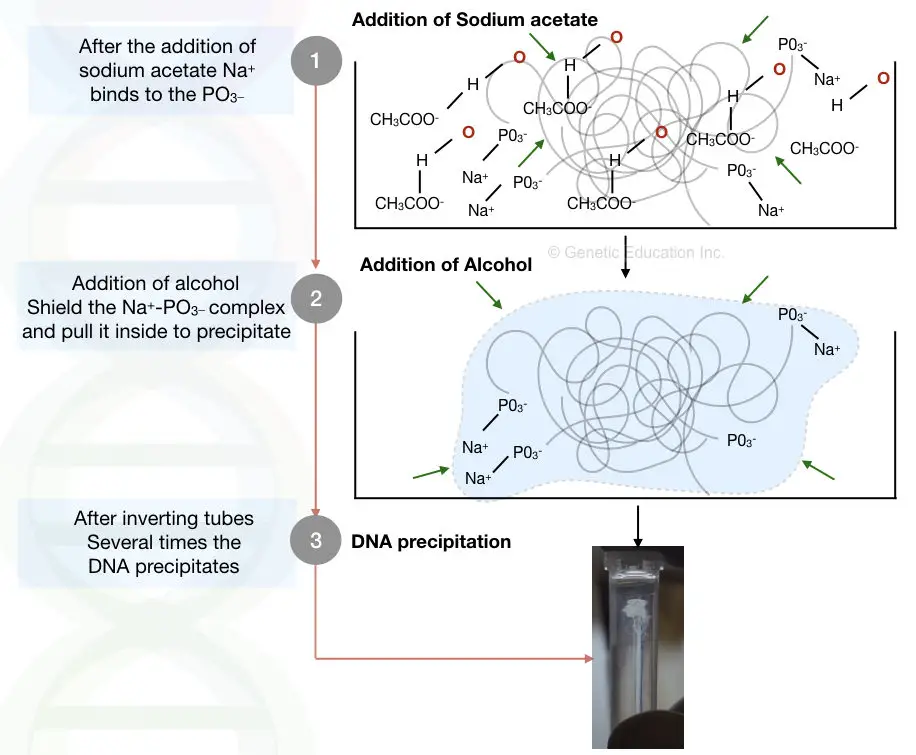
When we add salt for let’s say sodium acetate (CH3COONa+). The positively charged Na+ reacts with the negatively charged phosphate of the DNA. Now this reaction makes DNA less hydrophilic.
But water can destroy everything, the positive charge of water will try to dissolve and stabilize it. When we add alcohol, the net negative charge of alcohol makes the positive charge of water busy.
This disallows water to interact with the DNA. meaning, that alcohol prevents the Na and PO3– complex by neutralizing other positive charges. When we add more and more alcohol it neutralizes as many positive charges as possible from the solution and precipitates DNA in a visible form.
By the addition of a double volume of alcohol, almost all positive charges of water are neutralized by alcohol which pulls DNA out from the solution and eventually we can see a visible precipitate of DNA.
Usually, a doubled volume of isopropanol is used for precipitation in combination with sodium acetate salt. Ethanol can also be used. Do you know how precipitation occurs? Check out this article: A Quick Guide on DNA Precipitation and Protocol.

The actual image of DNA precipitate. ©Genetic Education Inc.
DNA washing:
Alcohol cleans DNA and removes other contaminants. Once again it uses the same principle. When we wash DNA with alcohol (by gently inverting the tube many times), alcohol passes through the DNA and removes proteins, salts and traces of other chemicals.
Centrifugation will remove the contaminants and by doing air-drying alcohol can be evaporated as well.
Usually, 70% ethanol is recommended to wash DNA precipitates. Three-time washing is advisable at 10,000 rpm centrifugation.
DNA storing:
TE dissolves DNA but over a period dissolved DNA can be degraded to some extent and sometimes, lose its properties. Adding alcohol to precipitate will make precipitate intact, prevent solubilization and thereby degradation.
1 to 1.5ml of ethanol (70%) is added to pellets and stored at -20°C temperature for years. The DNA will remain safe.
Which alcohol to use for DNA precipitation?
Now the experts part comes. You may wonder which alcohol from the isopropanol, ethanol and methanol. Here is the answer.
I personally do not recommend methanol. It is just an option you can consider but not useful. A doubled volume of ethanol and a single volume of isopropanol is used for precipitation. Notably, both should be 100% (absolute).
So the advantage of using isopropanol over ethanol is the low-volume requirement of the isopropanol. However, isopropanol can pro-precipitate more salts and also creates difficulties in DNA drying.
Technically, for 1 ml nucleic acid extract, you need 2 to 2.5 ml ethanol and 0.6 to 1 ml isopropanol.
| Alcohol | Concentration | Function |
| Isopropanol | 100% (Pure) | DNA Precipitation |
| Ethanol | 100% and 70% | DNA precipitation and DNA washing |
| Ethanol | 70% | DNA storage |
| Methanol | 70% | DNA washing |
Wrapping up:
These are three important functions of alcohol in DNA extraction. Precipitation is an important step here. The entire success of the experiment depends on how keenly you precipitated and washed DNA.
My technical knowledge also supports that chilled alcohol increases the yield of the precipitate. It is advisable to store isopropanol at -20°C before use and apply it in chilled form. Lower temperature prevents enzymatic degradation of DNA and thus we get high pure and high yield DNA.
FAQs:


Ice cold EtOH helps protects the DNA from DNases & increases the resultant yield. Also at low temperature, dissolution of DNA is prevented helping in more effective precipitation
What is the role of sodium acetate.. and how much quantity should added..
Please tell me sir..
Aashish Anant, Entomology PhD scholar
Sodium acetate is a salt used to precipitate the DNA. It increases the DNA precipitation yield. if you do not have it, you can also use sodium chloride.
Generally, a pinch of sodium acetate is enough or you can use 1/10th volume of the total supernatant.
Thanks for commenting.
Best wishes from Genetic Education.
Sir normally ethanol ko 70 % hi Kyu use karte Hai