“DNA precipitation is a process of nucleic acid (DNA/ RNA) precipitation using alcohol and salt. Ethanol and isopropanol are the two most common types of alcohol used in the DNA precipitation protocol.”
An important step of the DNA extraction protocol is DNA precipitation. DNA looks like cotton threads when precipitated. However, read-to-use DNA kits, used commonly in recent times, don’t need a precipitation step. It directly dissolves DNA in the elution buffer.
Traditional DNA extraction protocols rely on the precipitation step, it’s a kind of validation step, that indicates the presence of DNA. And that’s why scientists use this method so often.
Besides the precipitated DNA has lucrative applications too which we will discuss in this article. Alcohol is a common DNA precipitation agent, two common alcohol used in DNA extraction are isopropanol and ethanol.
A few common steps in the traditional DNA extraction protocol are
- Lysis of cell wall or cell membrane
- Lysis of nuclear membrane
- Isolation of nucleic acid
- Precipitation of DNA
- Washing of DNA
- Dissolving DNA
Related article:
Take a look at the figure below,
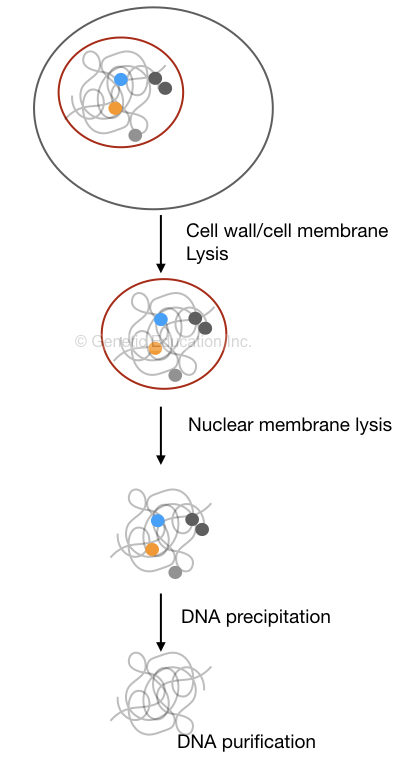
DNA precipitation is an amazing thing, it’s the only reaction in which we can visualize DNA by eyes, not like the spiral or helical structure explained in the literature.
If you are a student then it’s important for you to understand the process of how lysis, precipitation and elution occur during the process of DNA isolation. It will boost your DNA extraction knowledge trust me.
So in this article, I will explain to you the process of DNA precipitation and will give you a common DNA precipitation protocol that indeed helps you.
Stay tuned.
Key Topics:
What is DNA precipitation?
Alcohol precipitation precipitate or aggregate DNA into the visible cotton thread-like substance is known as the process of DNA precipitation
Definition:
“The precipitation is a process in which the reaction between solute and solvent creates an insoluble aggregate called precipitate and the process is called precipitation.”
The DNA is hydrophilic in nature which dissolves in water but not in alcohol.
The water has a partial positive charge and a partial negative charge. The positive charge of the water molecule interacts with the negative charge of the DNA (the PO3-) and dissolves it. However, the interaction is not so strong.
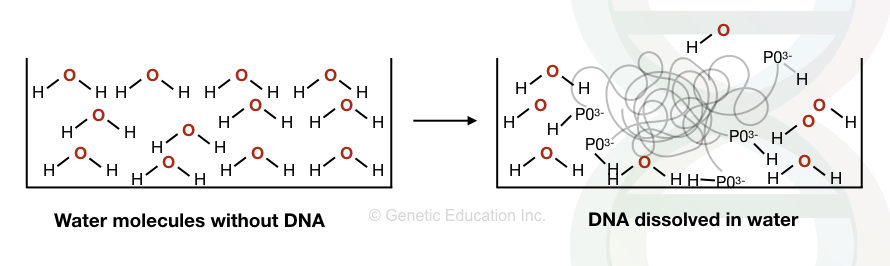
The salt and alcohol make the DNA more water hydrophobic. Once we add the salt into the DNA solution, the negative charge of the DNA interacts with the positive charge of the salt, instead of the positive charge of the water.
It’s possible for DNA to bind with water but because of the “lower dielectric constant alcohol”, the complex of DNA and salt is protected by an alcohol shield. And henceforth it can’t react with water.
This chemical reaction makes it possible to visualize DNA like a cotton thread and aggregates as a precipitate. For a more in-depth explanation of the mechanism of the DNA precipitation please read our previous article: Role of alcohol in DNA extraction.
In addition to this, the alcohol also does another job to increase the efficiency and yield of DNA precipitation. Instead of the negative charge of the DNA, the water molecule remains busy interacting with the negative charge of the alcohol.
The entire mechanism of precipitation is based on the polarity of the solution and the dielectric constant of the solvent.
Mechanism of DNA precipitation:
The DNA is a polar solution and so the water is. Therefore, by intermolecular interaction, the DNA (more precisely the nucleic acid) remains dissolved in the water.
(polar solution dissolve in polar and nonpolar solution dissolve in nonpolar solution)
Here, the interaction affinity is strong between the H+ and P03– of the water and DNA, respectively, hence both interact with each other. This interaction is necessary to separate DNA even into the next level of structure that is visibly separable.
A precipitate is a visible form of DNA (Although it does not look like the spiral DNA, it is just a clump of white aggregates), the final centrifugation step will separate DNA and remove impurities as well.
I hope you will understand what I mean to say.
Conclusively, precipitation separates DNA from other macromolecules present in the solution.
Now come to the point.
What does alcohol do?
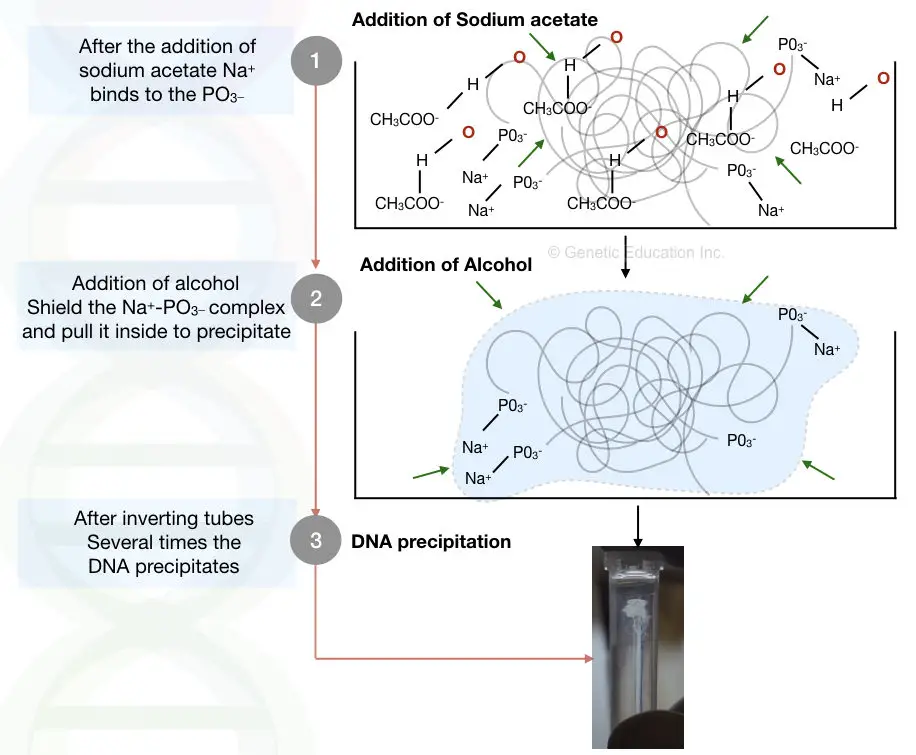
Here not only the alcohol but some of the salts such as sodium acetate, ammonium acetate, sodium chloride and lithium chloride perform a significant role.
The alcohol guards the “salt – DNA interaction”, if it is not shielded, the precipitates will not form.
The higher dielectric constant of water repels interactions of positive ions of the salt and negative ions of the DNA so it is very difficult to dissolve DNA only using salt.
By adding the alcohol such as ethanol and isopropanol, the lower dielectric constant of the alcohol protects the complex from water, and as we shake more the tube more positive charge of salts and negative charge of the DNA interacts with one another and we can get more precipitate.
Take a look at the pictorial representation here,
Read more articles related to this topic,
- CTAB DNA extraction buffer for plant DNA extraction
- Proteinase K DNA extraction method
- Phenol chloroform DNA extraction method
Importance of DNA precipitation:
DNA precipitation is used to precipitate DNA into a visible solid form.
It removes impurities from the DNA.
It can also be used to store DNA in dried form, precipitated DNA can be stored for a longer period of time.
Chemicals used in the DNA precipitation:
We had already given the name of all the chemicals used in the DNA precipitation. Some of the common salts used in the DNA precipitation are,
- Sodium acetate = 0.3 to 2M
- Sodium chloride = 0.2 to 0.3M
- Ammonium acetate = up to 5M
- Lithium chloride = 0.10M
Lithium chloride is the best choice for RNA precipitation but it is as good as sodium acetate for DNA precipitation.
Ammonium acetate is one of the choices but not recommended for all types of DNA samples.
Sodium chloride and sodium acetate are the two best choices for DNA precipitation in which I personally advise using sodium acetate.
Alcohol used in the DNA precipitation:
Alcohol is a key ingredient in not only DNA precipitation but also in DNA washing and DNA storing. Two commonly used alcohols are isopropanol and ethanol.
You may wonder why not methanol or other alcohol? Why only ethanol or isopropanol is used?
Well, the DNA precipitation mechanism is solely dependent on the dielectric constant of the solvent.
Let’s go through some of the numbers:
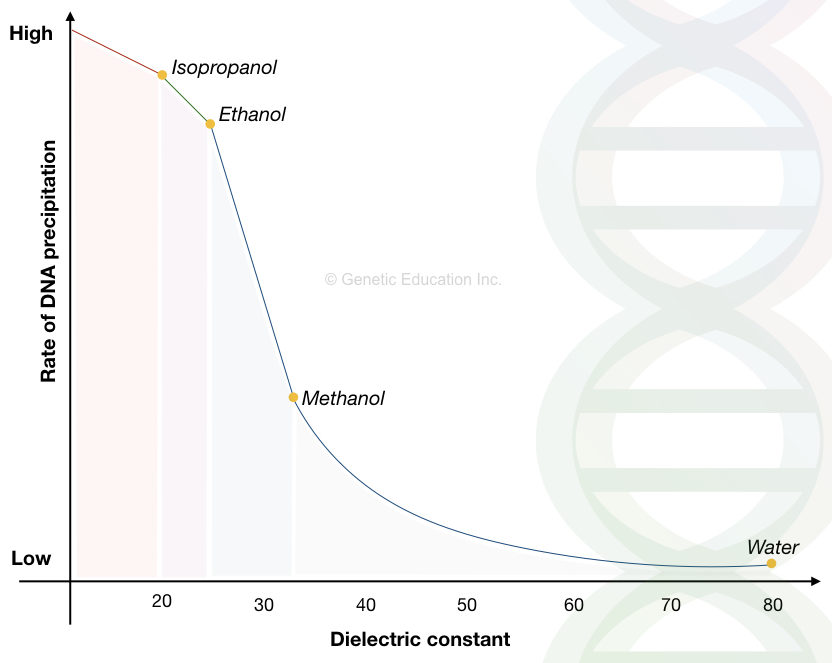
| Solvent | Boiling point (°C) | Dielectric constant |
| Water | 100 | 80 |
| Methanol | 68 | 33 |
| Ethanol | 78 | ~24.3 |
| Isopropanol | 98 | ~20.1 |
Take a close look at the methanol, the boiling point of methanol is 68°C and the dielectric constant is 33.
The boiling point of methanol is lower than the ethanol and isopropanol while the dielectric constant is higher. The lower the dielectric point, the higher the precipitation efficiency. We have already discussed why.
Furthermore, the Boiling point of both the champions is higher, which means it is safer to use.
In addition to this, methanol is toxic to us, therefore, Isopropanol and ethanol are the two best choices for DNA precipitation.
However, if needed we can use methanol, it is totally fine and works nicely (but not good for plasmid DNA extraction).
Use 95% alcohol in DNA precipitation.
So the conclusion of the story is, use isopropanol, it precipitates best. But wait, is there any problem with it?
Practically, the isopropanol also precipitates the salt too, which means that impurities still remain in our DNA and as we knew that the salts are the most dangerous inhibitors in the PCR reaction.
So, using ethanol is a wise decision for our DNA precipitation.
A common DNA precipitation protocol:
DNA precipitation is a subsidiary step in the DNA extraction process, when performed correctly will always give the best purity and yield of DNA.
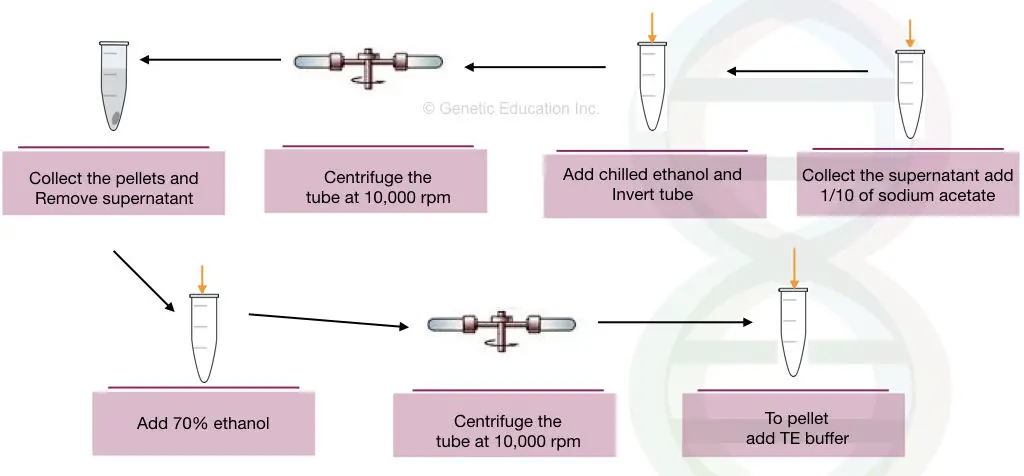
Let us start our protocol using two of the best chemicals for DNA precipitation, ethanol and sodium acetate.
- Prepare the sodium acetate solution of 2M at pH 5.2.
- Add the 1/10 volume of sodium acetate to the nucleic acid lysate solution.
- Add a doubled volume of pre-chilled ethanol.
- Invert the tubes several times gently to precipitate the DNA.
- After DNA precipitates appear (see the figure below) centrifuge the precipitated DNA at 10,000 rpm for 2 minutes.
- Remove the supernatant carefully without disturbing the pellets.
- Dry the pellets with tissue paper or air dry.
- Now add 70% alcohol and wash the sample by inverting it several times.
- Centrifuge the sample at 10,000 rpm for 2 minutes.
- Remove the alcohol and air dry the pellets.
- Once the pellets dry properly, dissolve them in the TE buffer at a pH of nearly 8.2.
Why is chilling necessary for DNA precipitation?
The process of flocculation is very important for obtaining a higher yield of the precipitate. The flocculation is achieved by chilling the sample.
At lower temperatures, high “flok” or aggregates are obtained by the addition of some of the precipitating agents.
The addition of chilled ethanol facilitates higher flocculation in the process viz the more DNA precipitation.
In some other protocols, if precipitates are not achieved, the sample is incubated at the lower temperature (-20°C) or in the liquid nitrogen for some time to achieve precipitation.
It is a very good practice for plasmid DNA or bacterial DNA precipitation.
Pro-tips:
For low DNA concentrate use 10 to 20 micrograms of glycogen and incubate the sample overnight at low temperature to increase the yield of DNA precipitate.
or
Add 10mM of MgCl2 to the precipitate and incubate for 1 to 1.5 hours at -20°C before centrifugation.
Both modifications greatly increase the yield of DNA precipitation for smaller fragment nucleic acid or low concentrate nucleic acid.
Notedly a double quantity of ethanol is required to precipitate the DNA.

For plasmid DNA or low concentrate DNA use this DNA precipitation protocol:
- Prepare the sodium acetate solution of 0.5M at pH 5.2.
- Add the 1/10 volume of sodium acetate to the nucleic acid lysate solution.
- Add a doubled volume of pre-chilled ethanol.
- Invert the tubes several times gently to precipitate the DNA.
- Add 10mM of MgCl2 and incubate at -20°C for 1 hour.
- Centrifuge the DNA precipitated DNA at 10,000 rpm for 2 minutes.
- Remove the supernatant carefully without disturbing the pellets.
- Dry the pellets with tissue paper or air dry.
- Now add 70% alcohol and wash the sample by inverting it several times.
- Centrifuge the sample at 10,000 rpm for 2 minutes.
- Remove the alcohol and air dry the pellets.
- Once the pellets dry properly, dissolve them in the TE buffer at a pH of nearly 8.2.
- If not dissolved properly, heat the sample at 60°C until it dissolves.

Tips: When you are using the isopropanol, do not freeze the sample or do not follow the chilling method because it precipitates more salts along with DNA.
Conclusion:
The use of manual or traditional DNA extraction protocol is quite uncommon, nowadays; however, the manual method saves cost.
Precipitation allows us to visualize DNA, it is a process, like magic; a moment ago you see nothing but when you shake it you can see DNA. That’s an amazing experience, trust me.
If you are really interested in pursuing your career in genetics, you should learn these things. Achieving highly pure DNA using manual methods is an art. Timely, when you do this repeatedly you will get expertise in it.
I highly recommend you to go through this technique, do it yourself, it will enhance your knowledge and skills.


