“Hot start PCR is a modification of conventional PCR in which limiting one of the reagents until the heating step reduces the chances of non-specific amplification.”
Non-specific binding or non-specific amplification is the biggest problem in any PCR (Polymerase Chain Reaction) reaction and leads to false results. Primer-dimers and binding at the non-target location are common reasons that cause non-specific amplification.
Technically, you may get many separate bands but you don’t know which one is your target product. There are many reasons for such conditions to occur in the PCR, for example, inappropriate annealing temperature, false primers, longer primers and too much time in reaction preparation.
Basically, primers can be re-prepared and annealing temperature can be re-set for new reactions, but what about the reaction preparation. Beginners and experienced lab personnel take their time to prepare a PCR reaction.
So between the time of reaction preparation and putting tubes into the thermocycler causes non-specific binding thereby non-specific amplification. Such factors are hard to identify and overcome. But on the other extreme, it is also important to take time to prepare the PCR reaction accurately.
You may wonder what to do. I have an answer.
What if we restrict the activity of any of the components of the reaction? For example, if the Taq DNA polymerase is there in the reaction but can’t polymerase early, before initiation of the heating step.
We can avoid non-specific amplification.
That’s what the hot-start PCR can do. Stops one of the components early during the reaction preparation. In the present article, I will explain to you the concept of hot-start PCR and how it is used during the PCR.
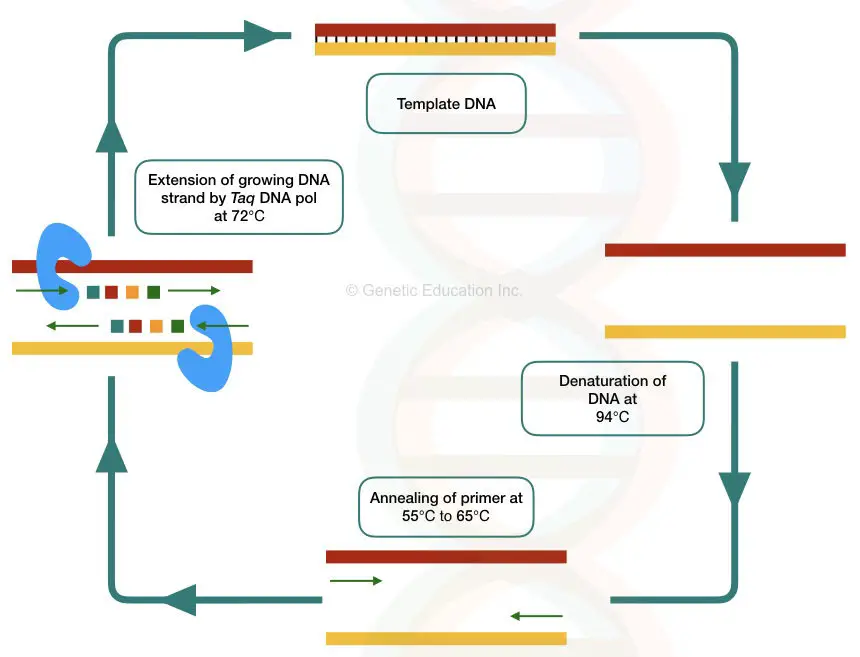
Key Topics:
Reason for non-specific binding:
As aforementioned, non-specific binding is a common PCR problem. Here I have explained several common reasons for that.
The annealing temperature:
If the annealing temperature of the reaction is lower than its original annealing temperature, it results in non-specific bindings.
The specificity of the primer:
If the primer is not specific to the complementary region, is longer than advisable and contains very high GC-content, it binds at many locations in a sequence thereby causing non-specific amplification.
But if you want to learn how to design primers effectively, read this article by clicking the link.
The concentration of primers:
The concentration of PCR primers is yet another factor resulting in the same condition- non-specific binding. If the concentration of primers is too high, you know what will happen in the reaction. Ideally, 10 pM primer concentration is enough.
Higher concentration of PCR additives:
PCR additives are good, you know! Because it provides ease in the reaction and eventually has a positive impact on a reaction. But concentration matters! If the concentration of any of the PCR additives is too high, it may result in non-specific binding and non-specific amplification.
An early action of Taq DNA polymerase:
Reaction preparation time is a major factor in achieving excellent PCR amplification. But usually avoided. As the reaction preparation time increases, the chances of non-specific binding also increase. You know, Taq DNA pol can also work at room temperature. So if you are preparing a PCR reaction and wasting time talking with your colleague, your reaction has to suffer, definitely.
Your Taq DNA polymerase starts the polymerization. Because it has all the things to make a new DNA strand. The early activity of Taq DNA polymerase causes non-specific amplification.
When you are dealing with more sensitive and costlier experiments like DNA sequencing, such mistakes ruin everything. So concepts like hot start PCR or host start Taq DNA polymerase are useful.
“Perform PCR reaction on ice.”
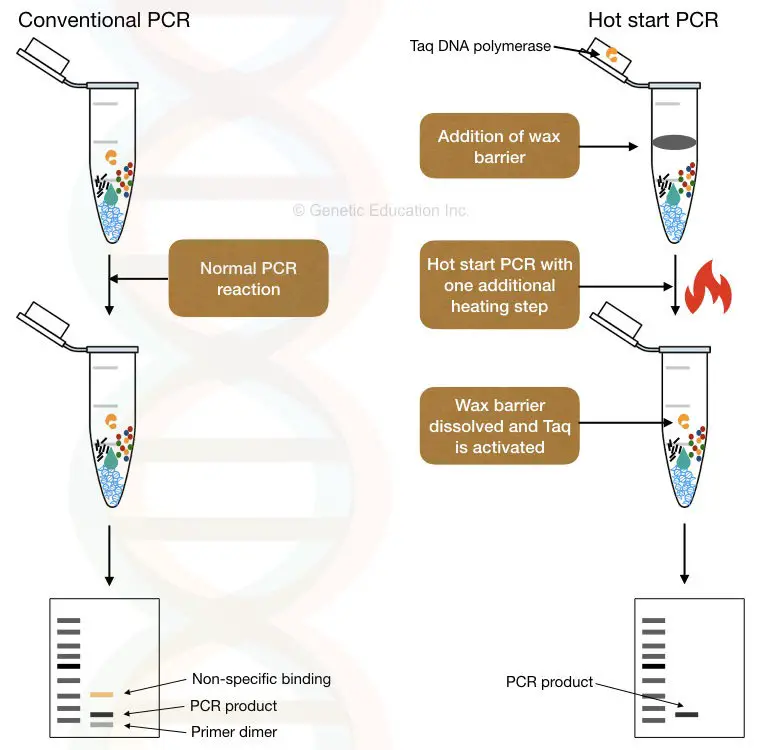
What is a hot-start PCR?
Hot start PCR is an excellent optimization of the PCR in which one of the reaction components will activate only during the heating step.
“ Hot start PCR = One of the components starts its activity under the hot condition of PCR.”
The main objective to perform a hot start reaction is to limit any of the components or Taq (specifically) thereby early amplification. Here are several ways using which the present goal can be achieved.
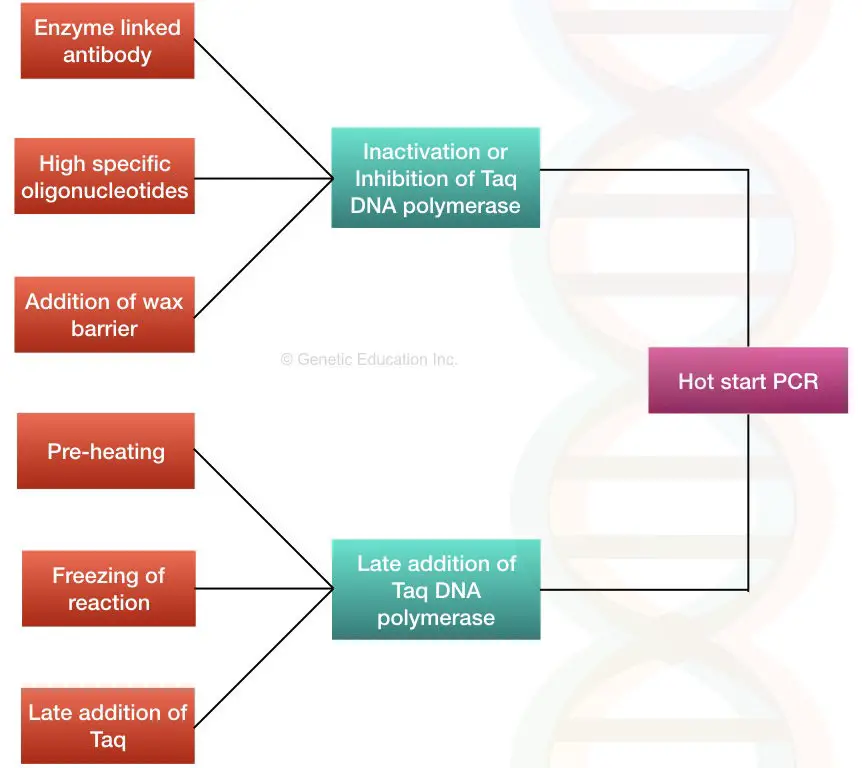
1. By using the enzyme-linked antibodies.
It is a very novel way to inactivate the Taq DNA polymerase early. Here, the polymerase is linked with the specific antibody which makes it busy. Hence it cannot amplify any DNA early before the reaction.
The antibody used here is temperature-sensitive; once the temperature reaches above 70°C, the antibody is released from the Taq DNA polymerase and starts polymerization.
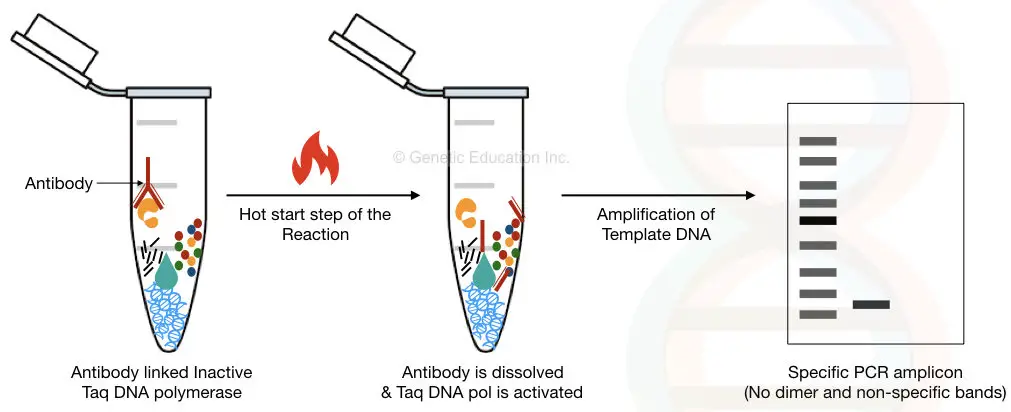
The limitation of the antibody-linked enzyme method is the cost of the reaction. Different enzymes need different antibodies so every time when we perform separate experiments the overall cost of the reaction will increase.
2. Preheating the PCR machine.
In this method heat the PCR machine at 95°C before reaction preparation. Prepare all the reactions on the ice at 4°C and immediately transfer them to the PCR. Eventually, Taq DNA pol can’t initiate catalysis early and prevent non-specific amplification. Research also suggests that preheating techniques release mispaired primers.
3. Freezing the PCR reaction.
Another method is deep-freezing the PCR mixture. Once the dNTPs, primers, water and template are added to the reaction, immediately the reaction mixture is frozen. After that, the Taq DNA polymerase and PCR additives such as MgCl2 are added to the frozen surface of the reaction.
Tubes are placed in the PCR.
Such practices prevent non-target amplification, primer-dimer and early polymerization.
4. The addition of Taq DNA polymerase separately.
Another wise decision is to add the polymerase separately. The reaction without the Taq DNA polymerase is prepared and placed in the PCR machine, once the machine achieves adequate temperature to synthesize DNA, the Taq is added to reaction tubes.
It is an excellent option, nonetheless, opening thermocycler, and PCR tubes and adding Taq in-between increases the chances of cross-contamination and reaction failure.
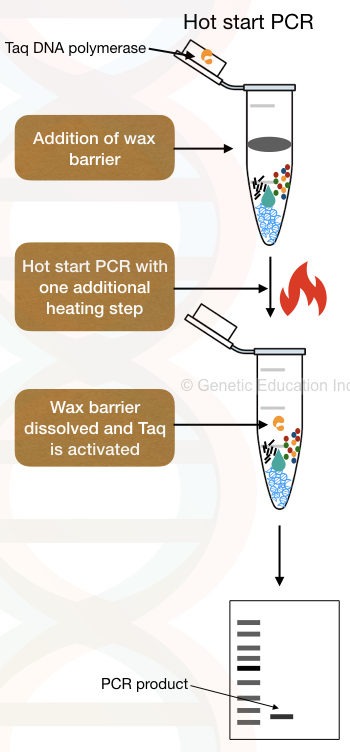
5. The use of Wax beads.
Widely-used, optimized and accurate hot start modification is the use of wax beads. Temperature-sensitive wax beads make a physical barrier between Taq DNA polymerase and other PCR components.
Generally, the Taq containing wax beads are placed inside the ‘tube-mouth’; PCR ingredients are added at the bottom of the tube.
In another method- wax beads without Taq, the Taq DNA polymerase and MgCl2 are added to the surface of the beads. Once the PCR achieves adequate temperature to melt the wax beads, Taq DNA polymerase incorporates in the reaction, immediately starts synthesis and prevents early amplification.
6. High specific oligonucleotides.
The use of high-specific oligonucleotides makes Taq busy doing nothing below the temperature of 50°C, only when the temperature exceeds over, high-specific oligonucleotides are detached from the enzyme.
Taq eventually enters late in the reaction and immediately starts amplification.
These are some of the options and optimizations for hot-start PCR, however,enzyme-linked antibodies, beads and high-specific oligonucleotides are excellent, well-established and accurate techniques.
The chances of false results are very high in amplifying the 80 or 90bp fragments as those fragments are very hard to distinguish from the non-specific amplification and primer-dimer extensions.
TB-PCR-like techniques highly rely on the hot start approach as it is more contagious. The sample is directly added with other reagents excluding Taq. The prolonged denaturation step will burst the bacterial cell membrane as well as activate Taq in the reaction.
Note that Taq is added in the form of antibody-linked or wax beads. This method is rapid, accurate and specific, I personally used it in my lab.
Advantages:
- The present technique decreases the chances of non-specific amplification.
- It prevents mispriming and primer dimer formation.
- The hot-start PCR technique decreases the nonspecific bindings.
- The reaction can be prepared at room temperature. No extra care is required.
- It increases the yield and accuracy of amplification
- Also, it prevents mispriming and primer dimer formation.
Disadvantages:
- The technique is costlier and tedious.
- It can’t be used for a longer DNA template more than 2Kb.
- A higher temperature for a prolonged time will damage the template DNA badly.
Hot Start Taq DNA polymerase:
The important player of the Hot start PCR technique is obviously, the HotstartTaq. The Hot start Taq DNA polymerase is different in comparison to the normal Taq DNA polymerase.
Commercially available all the Hot start Taq are chemically modified.
The hot start Taq DNA polymerases are either enzyme-linked, oligonucleotide-linked or chemically modified inactive enzymes. However, the chemistry of the Hot start Taq varies from manufacturer to manufacturer and they never reveal it.
From the 200U stock, 2U of hot-start Taq is sufficient for a 30μL reaction.
The commercial kit contains the 10X reaction buffer with MgCl2, KCl and other additives. Use a 1X buffer. Such ready-to-use kits increase the specificity and accuracy of reactions.
Conclusion:
PCR is amazing and a must-needed tool in molecular genetics. However, various modifications are recommended to achieve various goals. Non-specific amplification is the biggest and commonest problem of PCR. The hot-start PCR concept is a highly optimized option to overcome such problems.
Restricting early activation of Taq is the best and most reliable option to prevent the early reaction. Wax beads use is my favorite technique, I personally found it easy to use.
If you care about your PCR amplification and can’t achieve excellent sequencing data, use only the hot-start approach for amplification.


amazing
Thank you Sarwat
Really thank you a lot for providing such a clear and brief lesson about hot start pcr and its core principles i hope other related lessons of molecular biology are putten in this way..
Thank you for appreciating our work