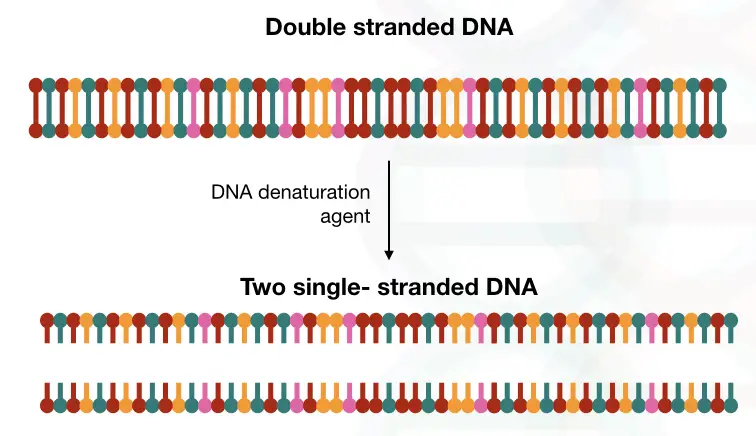
“DNA denaturation is a process to break the double-stranded DNA into single-stranded by either physical or chemical agents is known as DNA denaturation.”
For the last couple of weeks, we are exploring the PCR and related optimization things. So far we have discussed the importance of GC content, annealing temperature, qPCR protocol and asymmetric PCR.
We are going more in-depth, to understand the concept of PCR and denaturation more appropriately and that’s why I think this topic is important to discuss, the denaturation of DNA.
The in vitro or in vivo synthesis of DNA needs various enzymes, cofactors and a primer, however, primitive things for initiation of the process is to break open the dsDNA into two single-stranded ones.
The natural form of DNA at least for us is the dsDNA, acronym as double-stranded DNA. Two single DNA strands are joined by hydrogen bonds and that’s what the stable form of DNA is!
When we talk about the in vivo synthesis, during replication, DNA polymerase needs a single-stranded DNA to initiate the process of replication. The same is required for in vitro processes such as PCR, DNA sequencing or microarray.
However, the enzyme helicase can finely do the job during replication, the same isn’t possible for in vitro studies. Enzymes can’t work at higher temperatures or outside cells.
But as the hydrogen bonds are enough strong to hold two separate DNA strands, we need more energy to break or separate single-strands of DNA.
In the present article, I will explain the importance of DNA denaturation and various techniques to do this. I hope this article will help you in your PCR and other assay optimization.
Stay tuned.
Key Topics:
What is DNA denaturation?
In general the process of “denaturation” or “denaturing the biomolecule” encourage losing the structure. For example, denaturation of protein or DNA loses various levels of structure.
Denaturation and renaturation are a natural phenomenon that allows the synthesis of new nucleic acid. It’s useful in in vitro studies as well.
Definition:
The process in which a dsDNA becomes ssDNA using either chemical or physical agents is known as DNA denaturation.
Importance of denaturing DNA:
Now you may wonder why we need to denature the DNA?
Denaturing DNA allows us to amplify and hybridize DNA in various downstream assays. And helps in biological studies.
By denaturing the DNA, stability, properties, structural variation, sequence variation, concentration, expression and nucleotide sequence of the DNA can be studied and investigated.
The amplification helps in studying mutation and investigating various genetic and gene disorders while hybridization helps in studying and investigating gene expression and related studies.
Sequence variation can also be encountered using the denaturation-based assay and therefore we can say the whole genetics and genomic studies rely on the process of denaturation.
It’s an important step in PCR, DNA sequencing and DNA microarray study.
Mechanism of DNA denaturation:
The denaturation process break opens two antiparallel DNA strands using either chemical or physical agents. DNA has four bases A, T, G and C. The A and T are joined by two and G and C are joined by three hydrogen bonds.
A denaturation agent breaks hydrogen bonds between two strands and blocks renaturation temporarily or permanently. However, it is notable that denaturation can’t affect or break the phosphodiester bonds between adjacent nucleotides.
List of DNA denaturation agents:
Physical agents: Heat, UV light, high pressure, sonication and surface action.
Chemical agents: Acids, alkalis, heavy metals, DMSO, NaOH, guanidine detergents, salts urea. Formamide, DMSO, Guanidine, sodium salicylate, propylene glycol, urea and NaOH.
Techniques to Denature DNA:
Denature DNA by heat:
One of the most common techniques to denature, (theoretically or more precisely) to unwind the dsDNA is by heat. And is commonly practiced in routine lab experiments. At higher heat or temperature >90℃, hydrogen bonds between dsDNA break and produce two separate single strands.
However, cooling at room temperature promotes renaturation too. The heat denaturation process is simple, straightforward and doesn’t include chemicals henceforth commonly employed in the polymerase chain reaction (PCR).
Notedly, the heat denaturation process is less accurate and so doesn’t advised for DNA sequencing like experiments. Heat denaturation is usually completed at 90 to 98℃.
Denature DNA by high pH:
pH is yet another technique using which the dsDNA can be separated. The dsDNA remains stable between the pH ~7.0 to 8.0. So each chemical used and prepared for DNA extraction and PCR reaction is usually processed at this pH.
DNA is highly pH-sensitive. At pH, >9.0 or higher than 11.0 causes DNA denaturation, precisely. It indeed interferes with the base pairing, meaning hydrogen bonding. pH-dependent denaturation is commonly not practiced.
Denature DNA by Alkaline:
DNA denaturation by Alkaline- NaOH is the safest, accurate and rapid among all available techniques. One can get the ssDNA within a minute. Nonetheless, as the concentration decreases the time for denaturation also increases.
NaOH is the best choice to denature DNA during sensitive experiments such as DNA sequencing. Yet another important benefit of using it is it can allow renaturation, which other chemical agents can’t.
Using the phosphate buffer the dsDNA can be recovered. To achieve various denaturation objectives varied concentrations of the NaOH can be used, ideally are 0.01, 0.1 and 1 mol/L.
The stock solution of 10 mol/L is prepared by adding 40 g of NaOH pellets to 100 mL distilled water.
Denature DNA by formamide:
The formamide is commonly used in molecular genetic experiments to lower down the melting temperature of the DNA during the amplification. Previous studies suggest that a 10% increase concentration of the formamide can decrease the melting temperature up to 7.2℃.
Wang X, Lim HJ & Son A, (2014) suggested that 50% formamide can decrease the melting temperature up to 10℃. Meaning, it has a lower denaturation capacity, notedly the major uses of formamide are as an RNA stabilizer or storing and preserving biological specimens and samples.
It can lower down the annealing temperature to denature the DNA, however, less data is available in support of the direct use of the formamide to denature DNA.
Denature DNA by DMSO:
Much like the formamide, the DMSO (Dimethylsulfoxide) is a proven melting temperature lowering agent. As the concentration of DMSO increases, the melting temperature will decrease.
Previous studies also suggest that 20% DMSO can decrease the melting temperature by as much as 15 to 20℃. Notedly, the direct use of DMSO to denature the DNA isn’t validated yet. Conclusively, the DMSO can lower down the denaturation process.
Blake RD & Delcourt SG, (1996) suggested that 50% DMSO can do denaturation during the PCR within 1 minute. Although as the concentration increases the denaturation capacity decreases.
Denature DNA by salts:
Salts are the strong DNA denaturation agents that can permanently denature the DNA (Which can’t be renatured). Salt along with other organic solvents or physical processes like heat denaturation can effectively do the job.
However, as it’s a kind of permanent damage to DNA, usually salt-based denaturation is discouraged. But notedly, a controlled concentration of salts can be useful in many ways, for example, the 1/10 volume of salts like NaCl along with the alcohol can precipitate the DNA effectively.
Denature DNA by urea:
Urea is a powerful agent for denaturing DNA, commonly known as chaotropic denaturants which can denature DNA and protein. It can work as a hydrogen bond donor and acceptor and so break and make hydrogen bonds between two strands of DNA.
Ideally, 6 to 8 M urea is used in the process like PAGE to separate DNA molecules.
Applications of DNA denaturation:
Many different assays use the “process” DNA denaturation for various purposes and studies.
PCR: In the PCR, it helps in investigating mutations and discriminating alleles.
DNA sequencing: DNA sequencing separates different polynucleotides based on the sequence variation.
DNA microarray: Denaturation promotes probe hybridization and helps in gene expression studies.
Southern blotting: Denaturation allows binding of the probe to validate the presence or absence of nucleotide sequence.
Agarose gel electrophoresis: Denaturation allows easy migration of ssDNA in the gel and helps to determine the size of the DNA, especially in the PAGE.
Wrapping up:
Unlike NaOH, the majority of chemical denaturants work in PCR with heat, meaning it alters the melting temperature. Denaturing DNA has significant importance in the downstream application, however, to get good results, DNA should denature completely.
Besides, heat, the common denaturation agent is the NaOH, which works by changing the pH of the DNA.
Sources:
Wang X, Lim HJ, Son A. Characterization of denaturation and renaturation of DNA for DNA hybridization. Environ Health Toxicol. 2014;29:e2014007. Published 2014 Sep 11. doi:10.5620/eht.2014.29.e2014007.
Blake RD, Delcourt SG. Thermodynamic effects of formamide on DNA stability. Nucleic Acids Res. 1996;24(11):2095-2103. doi:10.1093/nar/24.11.2095.