“T4 DNA ligase is a commercially available ligase that is used in gene cloning, recombinant DNA technology and genetic engineering experiments. Here is a short note on T4 DNA ligase.”
In the previous article of the replication series, we discussed what DNA ligase is and how it helps in replication. Put simply, ligase is an ATP-dependent DNA enzyme that joins two DNA strands through a phosphodiester bond.
In genetic research, we often need to join two nucleic acid strands. For example– insertion of the target DNA sequence into the plasmid. Along with other enzymes, we need ligase enzymes for this kind of experiment.
T4 DNA ligase is widely used in genetic engineering, cloning and recombinant DNA technology experiments. In this article, I will introduce T4 DNA ligase to you and provide you with some crucial information on it.
Stay tuned
Disclaimer: Information provided here is collected from peer-reviewed resources and re-presented in an understandable language. All the sources are enlisted at the end of the article.
Key Topics:
What is T4 DNA ligase?
In 1983, Armstrong and coworkers discovered the structure and genetic organization of T4 DNA ligase, however, the first T4 DNA ligase was cloned by Wilson and Murray in 1979.
It’s an ATP-dependent DNA ligase that requires an energy molecule for catalytic action. It is extracted from the T4 bacteriophage virus. Usually known as ‘phase,’ bacteriophage infects E. coli. Using the present ligase we can prepare a covalent bond between both blunt and sticky ends generated by restriction digestion.
Properties:
| Protein Accession | P00970 |
| Gene | Gp30 (gene 30) of T4 bacteriophage |
| pH | 7.8-8.0 |
| Molecular weight | 77 kDa (Sedimentation, Lehman 1974)74 ± 3 kDa (SDS Page, Lehman 1974)55.3 kDa (Theoretical) |
| Isoelectric point | 6.14 |
| Activators | ATP, Mg2+, sulfhydryl reagents |
| Optimum temperature | 16°C |
| Inhibitors | A higher concentration of NaCl |
Like the natural DNA ligase, T4 DNA ligase also contains a nucleotide-binding site, a cleft and a lysin-reach active site. Notedly, it uses the same mechanism as other DNA ligases. The mechanism of action is explained in our previous article. You can read there.
Maximum activity of T4 ligase can be achieved below the body temperature, between 16 to 22ºC temperature. As the temperature increases, the activity decreases. Check out this graph.
Function of T4 DNA ligase:
T4 DNA ligase can not only ligate two DNA molecules but also DNA-RNA, plasmid and DNA, and even RNA-RNA molecules during the experiment. However, it’s important to note that it can’t join single-stranded nucleic acid, effectively.
Applications of T4 DNA ligase:
The present enzyme is widely used in gene cloning and recombinant DNA technology. For example, if we wish to transfer our gene of interest into a cell line. What we can do is, use a recombinant plasmid.
We digest the plasmid and our GOI (gene of interest), join both the DNA molecules and in the final step we introduce the plasmid into target cells. Now, for inserting a GOI or target DNA sequence into the plasmid, we need to seal them.
T4 DNA ligase is capable enough to catalyze such reactions and ligate plasmid insertion.
In experiments that need linker DNA, T4 DNA ligase is widely used. Linker DNAs are like a known sequence tag which we use into sequence for validation purposes. Linkers are incorporated into the sequence using the T4 DNA ligase.
In DNA damage and repair studies, T4 DNA is utilized to fill gaps between two DNA molecules.
Most importantly, the present enzyme is widely used in library preparation. Libraries (cDNA or genomic DNA) are the collection of known sequences. Here known sequence adapters are introduced as a sequence tag. Libraries are prepared for DNA sequencing experiments.
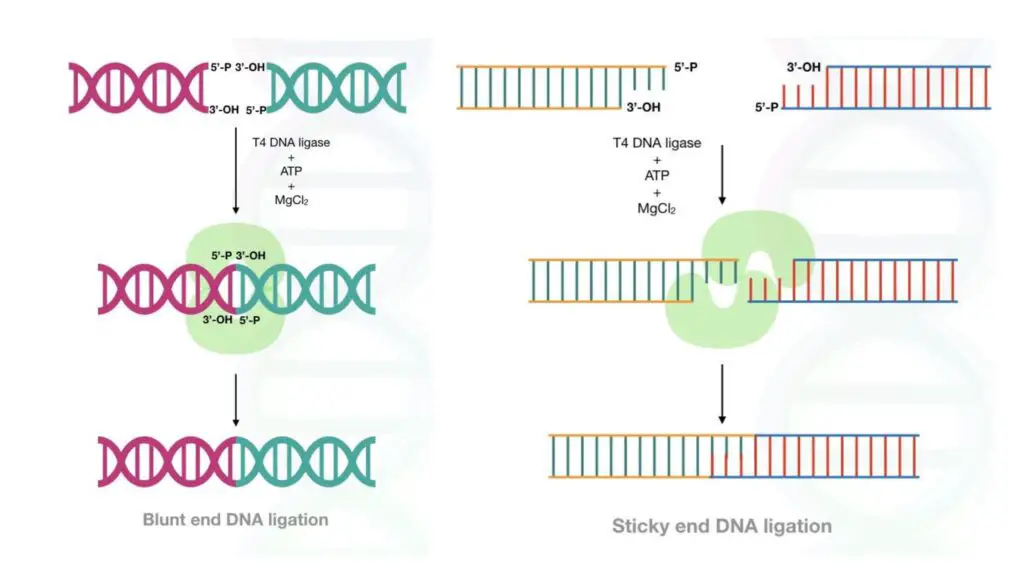
Blunt ends and Sticky ends joining:
Blunt ends and sticky ends are generated during the restriction digestion process. Blunt ends are generated without any sequence overhang, meaning, ligase has to join two DNA fragments end-to-end.
Sticky ends possess a few nucleotide overhangs on both sequences. In both cases, the ligase prepares phosphodiester bonds using the ATP as an energy.
Limitations:
Despite its wide utility and popularity in molecular genetic research and experiments, T4 DNA ligase also has some limitations.
- Studies show that it can’t seal single-stranded DNA effectively. It requires a stable-duplex structure to settle and perform catalytic activity.
- It requires a co-factor (ATP) to execute its activity.
- It’s temperature-sensitive. As it works at a lower temperature, a slight fluctuation in temperature adversely affects the reaction.
T4 DNA Ligase Protocol:
First, prepare a reaction buffer by adding the reagents given in the table above. Store the buffer in an adequate condition.
Recipe for ligation buffer preparation:
| Tris-HCl | 500mM |
| MgCl2 | 100mM |
| Dithiothreitol | 100mM |
| ATP | 10mM |
*Adjust pH 7.0 and store it in a sterile place.
The protocol for the T4 DNA ligase is very simple. Check out the table below first.
Ligation reaction preparation:
| Requirement | Concentration |
| Insert DNA | 100 ng |
| Plasmid DNA | 30ng |
| T4 DNA ligase | 0.5 to 1μl |
| Ligation buffer | 10X |
*Add DD/W to make the final volume of 10μl.
Add 100 ng insert DNA, 30 ng plasmid and 0.5 to 1μl T4 DNA ligase.
Now add 10X reaction buffer. Reaction buffer provides cofactor and other essential reaction conditions.
Add DD/W to make the final reaction volume 10 μl.
Our reaction is ready now incubate it at 16ºC temperature or at room temperature, depending on the instructions given in the manual.
After the completion of the reaction, the ligation can be validated either using gel electrophoresis, PCR or DNA sequencing.
Important note:
The DNA ligation buffer for T4 DNA ligase contains 10mM part of ATP which provides two ATP energy molecules in each reaction.
The activity of ATP degrades upon repeated freeze/thawing. Avoid repetitive freeze/thawing cycles. Store buffer in various aliquots. Always store the buffer at -20°C to protect the ATP.
Ligation time and temperature are crucial. All the DNA ligases are generally active at room temperature, however, the optimum activity of DNA ligase is obtained at 16°C temperature (see the graph above). You can incubate the sample at room temperature for 2 hours or at 16°C overnight.
Interestingly, another commercially available DNA ligase called “Ampligase” DNA ligase can work efficiently even at a higher temperature of more than 95°C. The “ampligase” is isolated from the thermophilic bacteria having a half-life of 48 hours at 65°C and 1 hour at 95°C.
This DNA ligase can be stably worked for more than 50 thermal cycle reactions.
Wrapping up:
DNA ligase is significantly important for genetic experiments. T4 DNA ligase is an important commercial enzyme that we use in various experiments. Other ligases are now available which have amazing properties.
I am planning to prepare an article on various types of commercially available DNA ligases. I hope you like this article. Please share this article and bookmark the present page.
Sources:
Shi K, Bohl TE, Park J, Zasada A, Malik S, Banerjee S, Tran V, Li N, Yin Z, Kurniawan F, Orellana K, Aihara H. T4 DNA ligase structure reveals a prototypical ATP-dependent ligase with a unique mode of sliding clamp interaction. Nucleic Acids Res. 2018 Nov 2;46(19):10474-10488. doi: 10.1093/nar/gky776. PMID: 30169742; PMCID: PMC6212786.
Gaastra W, Hansen K. Ligation of DNA with t(4) DNA ligase. Methods Mol Biol. 1985;2:225-30. doi: 10.1385/0-89603-064-4:225. PMID: 21374196.
Subscribe to our weekly newsletter for the latest blogs, articles and updates, and never miss the latest product or an exclusive offer.