“Tissue homogenization is a process of preparing a homogenous emulsion or suspension of cells through chemical, physical, mechanical or other ways.”
RNA extraction is a ‘bone-crushing’ process and a headache for beginners, however, experienced players know how to tackle its (RNA) ego! RNA is, indeed, more fragile and sensitive than DNA, and also, constantly under attack by the RNase. Thus, it seeks special care and treatment.
I failed many times during my initial days when I was learning nucleic acid extraction. When we see bands in a gel, look at our guide and just tried to justify our work, she politely said, `That’s not RNA.’
Even without seeing a gel, she often said that that’s not RNA. It was literally painful, frustrating and annoying. We had made a checklist to make every step more effective but she knew we would get nothing. After many failures, she told us that the pre-preparation wasn’t satisfactory. She was talking about tissue homogenization.
So I and my other colleagues learned that taking care of RNA, RNase and contamination isn’t a ‘success guaranteed’ for RNA extraction. Effective homogenization increases the chances of getting excellent RNA yield by many folds.
If cells aren’t treated well and lysed thoroughly, everything, afterward, can’t work effectually. Not only for RNA, but the tissue homogenization process is also crucial for DNA extraction, especially for plant tissues. You know how hard it is to even isolate DNA from plant tissue.
Anyway, come to RNA Extraction.
The homogenization process lyses cells well and releases the intracellular components into the solution. So it makes post-processing by chemical treatment or enzymatic lysis even more impressive. But homogenization is required prior to cell lysis.
Note:
Lysis and homogenization are marginally different.
Even though you are in genetics, you probably know about homogenizing tissues by crushing using a mortar and pestle. But that… sometimes works but not always! So you may wonder how this works- the tissue homogenization “effectively”? What is the alternative technique or excellent one for RNA isolation?
In the present article, I will explain what tissue homogenization is, a practical approach to get success always in, and common methods you can try.
Stay tuned.
Hey, we have a whole series of articles on RNA extraction, you can read it here:
Key Topics:
Techniques for tissue homogenization:
Many ways molecular geneticists have to smash a tissue, some are good, easy to use and handy while some are tedious, extensive and costly. I will explain the techniques we use, but to make things more understandable, we will have to look at what various techniques are.
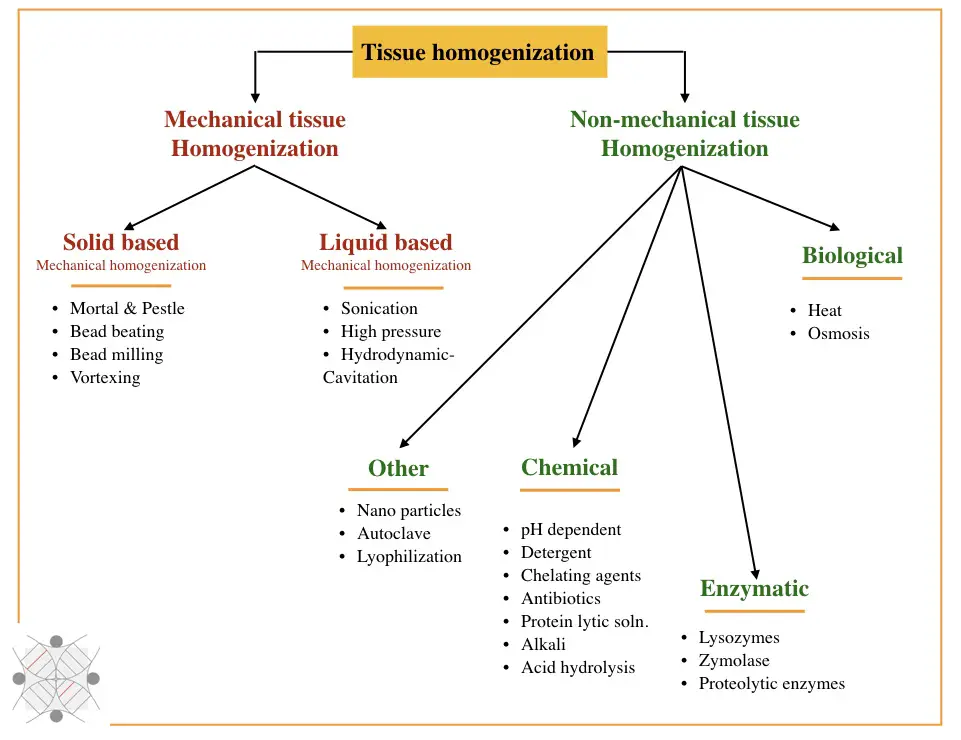
Largely, homogenization techniques are classified as mechanical or physical techniques and non-mechanical techniques. Mechanical techniques are further grouped into solid lysis techniques and liquid lysis techniques, whereas non-mechanical techniques are chemical, enzymatic and biological. Take a look.
Mechanical methods: Solid-based and liquid-based homogenization
Solid based mechanical homogenization:
- Mortar and pestle
- Bead milling
- Bead beating
- Vortexing
Liquid-based mechanical homogenization:
- Sonication
- High-pressure
- Hydrodynamic cavitation
Biological (Non-mechanical) techniques: Chemical methods, enzymatic methods and biological methods
Chemical homogenization:
- pH-dependent homogenization
- Detergent
- Chelating agent
- Antibiotics
- Protein lytic solutions
- Alkali
- Acid hydrolysis
Enzymatic homogenization:
- Lysozyme
- Zymolase
- Proteolytic enzymes
Other techniques
- Heat
- Osmosis
- Lyophilization
- Autoclave
- Nanoparticles
Several good techniques are here.
Mortar and Pestle- A conventional and convenient way:
The use of Mortar and Pestle to grind tissues for nucleic acid extraction is a conventional, traditional, affordable and cheap option. Especially, for plant tissues, it’s a better option. To extract RNA, liquid nitrogen is additionally used to maintain RNA integrity.
Liquid nitrogen is added to the tissues and ground vigorously, and quickly. To the powdery tissues add an RNA extraction buffer and collect them in an RNase-free tube. However, care must be taken while handling the liquid nitrogen and also allow it to evaporate completely.
All steps should be completed quickly. In comparison with other techniques, the use of mortar and pestle greatly increases the chances of RNase contamination. Also, additional homogenization steps are required to get a good yield.

Mechanical disruption:
Plenty of mechanical disruption techniques are available which rely on the use of a mechanical instrument, for example, a rotor-stator. Rotating blades grind tissues vigorously and can also homogenize cell culture. It can even handle a minimum volume sample.
A separate rotor-stator is required for mechanical disruption. It takes around 90 seconds to completely homogenize the tissue, nonetheless, the time depends on the type of tissue. Other mechanical disruption methods are bead milling, bead beating and vortexing.
Search ‘rotor-stator’ on Google and you will find what it looks like.
Vortexing:
Vortexing is the popular and effective cell lysis/homogenization technique. A tissue sample is taken into a cell lysis buffer and lysed using hard vortexing, followed by centrifugation to collect the nucleic acid.
Vortexing is safe, less prone to contamination and easy to perform. It can lyse any tissue type, notwithstanding, sometimes it even degrades the nucleic acid too.
Sonication:
Sonication is also a type of mechanical lysis (liquid-based). High-frequency sound waves pass through the sample and perform cell disruption under a liquid medium. The technique is tedious, time-consuming and costly.
I never used sonication so I can’t explain more about it.
Biological or enzymatic method:
Digestive enzymes like zymolase or lysosomes are also good lysis agents. Such enzymes have catalytic action to disrupt or digest cell walls, nuclear membrane and other proteins present in a cell. Note that enzymatic methods work excellently in combination with mechanical lysis.
Homogenization with mortar and pestle followed by enzymatic treatment is my secret to success in RNA extraction most of the time. Keep note that the enzyme should not affect the integrity of RNA. Biological techniques must be needed for tissues like plants or fungi.
These methods are common, and molecular genetic labs use them routinely. However, this information only helps you if you know which method is optimal for your tissue type, although I don’t know what tissue or cells you are working with.
So let us discuss the type of techniques used for different types of tissues.
Pro-tip:
Chill Mortar and Pestle on dry ice and use it for homogenization.
Frozen vs unfrozen tissues:
I personally don’t recommend processing frozen tissues, especially for RNA extraction. I faced problems, the yield isn’t good and the purity is a big concern and if the freezing isn’t performed well, it eventually results in RNA degradation.
Fresh tissues are good and give better results but in most cases, aren’t available. So cryopreservation is mandatory in RNA extraction. When the sample is collected far away from the lab and the sample load is huge, we can’t process samples immediately so we have to extremely freeze them. Take a look at the table below,
| Sample collected | Cryopreservation | Results |
| Within 24 hours | Stored at 4°C | Excellent |
| within a week | -20°C | Good |
| Within a month or more | -80°C in liquid nitrogen | Not so good |
Long story short, unfreeze, samples are good, easy to homogenize, and give excellent yield and quality of RNA while freeze tissues require extensive setup and care, have to homogenize and the yield and quality of the RNA aren’t so good.
Also, the RNA may lose its integrity, degrade or contaminate during freezing. So cryopreserved tissues aren’t advisable.
So tissue grinding, vortexing, and chemical and enzymatic homogenization techniques are, I think, excellent choices for fresh tissues, however, other high-end mechanical techniques, processes and instruments are required to homogenize frozen and hard tissues.
Up to this, still, the question is unanswered, the technique you require for your tissue type. Let’s find out the answer.
Bacterial cell or culture:
Bacteria broadly divide into gram-negative and gram-positive types and further have huge diversity, so “one size doesn’t fit all.” Bacterial cell suspension, or cell, in general, can easily be lysed using either chemicals or enzymes or both. Chemicals like SDS or beta-mercaptoethanol and enzymes like lysozyme work.
Note that no tissue grinding by mortar or pestle is required, however, gentle vortexing can improve the homogenization, greatly. For some ‘special’ bacteria, sonication and bead-beating techniques are used, if chemical processing doesn’t give adequate results.
Besides, bead milling which is rapid and safer (for RNA), also provides good quality RNA. Adding glass or silica beads, and lysis buffer to a tissue sample followed by milling for a few seconds prepares an exceptionally good sample for RNA extraction. I haven’t used bead milling yet.
Fungi:
Fungi, like the yeast, have hard cell coating like capsules that seek a special homogenization technique. Fungi is so diverse and so is its cell wall too. The composition of cell-wall varies between different fungi. In addition, the lysis buffer isn’t alone capable of completing the homogenization.
So which techniques to use to homogenize fungal cells depends on their species and cell wall composition. For fungi, alone vortexing, chemical treatment or enzymatic lysis doesn’t work and in addition, processing samples for different treatments increase the chances of contamination and RNA degradation.
So the one-stop solution is to use bead milling or bead beating. Both are mechanical techniques, safer, faster and more effective. So if your target sample is fungi, you need to require these two techniques in your routine.
Plant tissue:
Nucleic acid extraction from plant tissue is the most challenging and tedious task. Plants often have a high amount of polysaccharides, polyphenolic compounds and other secondary metabolites, such molecules are hard to remove.
Woody plants have a much higher amount of such chemicals. Furthermore, polysaccharides and polyphenolics can co-precipitate along with the RNA therefore, create chaos during extraction. However, oftentimes, tissue grinding will help to overcome such cumbersomeness. To remove polyphenols and polysaccharides use PVP (polyvinylpyrrolidone) in the sample.
Plant tissue homogenization doesn’t usually need a special setup, (though it might be required sometimes). In the process, we will have to take a tissue, add liquid nitrogen and grind it until it becomes powder. The co-product is further homogenized by adding the tissue or cell lysis buffer.
Following the lysis, collect cell suspension in a sterile tube, mix it, vortex it and centrifuge it.
We can use solicitation too, for further fine homogenization. The plant RNA extraction technique highly relies on tissue homogenization.
In addition, if plant nucleic acid extraction is a routine process in your lab, or if you require it timely, use mechanical techniques like bread beating and bead milling. My personal recommendation is for mortar and pestle + liquid nitrogen + lysis buffer.
Animal tissues:
Human, animal or clinical geneticists have to work with animal tissues such as liver, skin, bone, muscle, connective tissue, blood or cancer biopsy. Ideally, any animal tissue should be homogenized within 30 minutes of collection. and/or/otherwise, immediately cryopreserved in liquid nitrogen at a temperature below zero.
While working with RNA, store the sample in an RNA-protecting buffer (which is commercially available, I am talking about RNAlater, RNAsure, etc).
Every animal tissue has a different composition and thus needs a separate homogenization treatment. The protein foundation, polysaccharides and nuclease profile vary from tissue to tissue. It is also important to keep in mind that human tissues such as the spleen, liver, heart or brain are hard to homogenize as well as precious, therefore, care must be taken and a sincere approach is required during isolation.
I didn’t work with every animal tissue so I read some literature on how different animal tissues can be homogenized and presented them here. Brain tissues are rich in lipids like the plant so PVP (polyvinylpyrrolidone) is advisable to use.
Homogenize brain tissue in an animal lysis buffer, vortex it, centrifuge it and collect the supernatant. To improve the yield, use the rest of the tissue once again with the same process.
Fibrous tissues like the heart or skeleton muscles are one of the hardest tissues to isolate RNA. The reason is that it has low cell density as well as low RNA content. Freeze lysis using liquid nitrogen may help to improve the lysis yield.
Some tissue has a much higher amount of nucleic acids and nucleases, for example, rat spleen or thymus. Such tissues also required nuclease deactivation during homogenization. The use of the correct lysis buffer and aseptic conditions will surely boost the output. Furthermore, Cryo treatment and the use of liquid nitrogen are also advisable.
For human or animal tissues, I personally recommend using commercially available homogenizers.
So this is my experience with tissue homogenization, in recent times, there are way more options available that are effective, safe and cost-effective.
Read more: Different Types of DNA Extraction Methods.
Effective and proven tactics:
- Use only 10 to 50 mg of tissue, try the minimum volume of tissue but keep in mind that it should be in enough quantity to extract RNA.
- Use an appropriate amount of RNA extraction buffer, for example, 500µl per 10mg.
- Optimize the content of the lysis buffer. Use beta-mercaptoethanol, Guanidium thiocyanate, phenol and other required chemicals for effective cell lysis.
- Add an extra step of enzymatic lysis to digest, cellular and cell wall proteins.
- If required, use liquid nitrogen and homogenize vigorously. Take necessary precautions and follow liquid nitrogen handling guidelines.
- Always use sterile and RNase-free utilities.
- Follow standard guidelines for protecting the integrity of RNA.
- For hard materials like connective tissue, bone or plant bark grind to make a liquidy and homogeneous mixture until no visible particles or clumps appear.
- Freeze mortar, pestle, and small instruments on dry ice before use.
Wrapping up:
RNA extraction is an extremely sensitive technique and requires high-end expertise and experience. Though labs should have automated nucleic acid extractors, one should have to learn manual things. Trust me it is needed.
In case of auto-extractor maintenance, damage or validation, you should have to extract RNA manually. So nucleic acid extraction is a key skill every genetic student should have to learn. I hope this article will help you in your genetic studies.
Subscribe to our weekly newsletter for the latest blogs, articles and updates, and never miss the latest product or an exclusive offer.


