“A running buffer in electrophoresis is a liquid medium that helps proper migration of DNA or RNA during the electrophoresis run. Understand the significance and importance of a buffer in electrophoresis in this article.”
In the previous article of this series, I compared the TAE vs TBE buffer to choose for an experiment. That was a straightforward article to utilize for your experiment. However, it’s crucial to introduce the concept of a running buffer, at least for novices!
The electrophoresis technique is known to every geneticist or even life science student. It’s burdened with the glorious purpose of separating biomolecules. In our case, it is either DNA or RNA.
What we do is, we prepare a gel, pour it, place it in the tank and fill the tank with a buffer– a watery, crystal clear solution that contains some super important chemicals for electrophoresis. That’s our running buffer.
Now, at this point in time, you may wonder why we use it. What exactly does a buffer system do during electrophoresis and what options do we have? Let’s find out in this article.
But before moving ahead, if you are still unaware of electrophoresis, check out this article: Agarose gel electrophoresis— definition, Principle, Process, Protocol and Applications.
Disclaimer: Information provided here is collected from peer-reviewed resources and re-presented in an understandable language. All the sources are enlisted at the end of the article.
Key Topics:
Function of a running buffer in electrophoresis:
Besides maintaining the pH only, a running buffer, also known as electrophoresis buffer has 7 other crucial functions too. Here I have explained each purpose one by one.
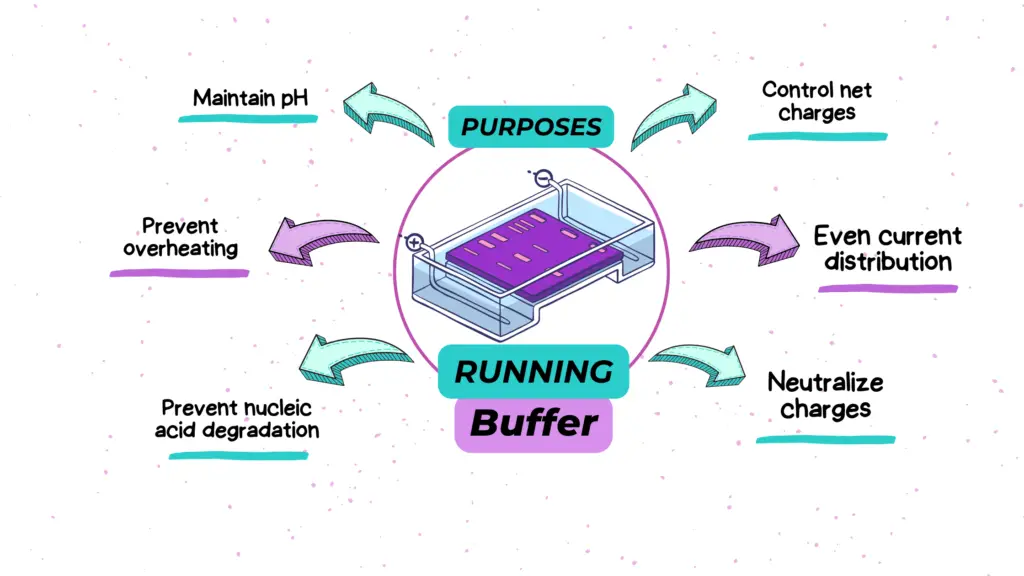
- Maintain stable pH:
The prime function of a buffer is to balance the ratio of weak acid and its correspondent conjugate base, and vice versa. We will not explain the chemistry behind the pH, as it is not the topic for discussion.
You just understand that for an electrophoresis run, we need a constant pH environment between 8.0 and 8.5 and the buffer having Tris, serves this purpose.
- Control net charges:
It also controls the net charges on DNA or RNA which eventually helps in proper migration.
- Maintain the temperature:
Electrical current increases the temperature during the run. Temperature increment results in nucleic acid degradation or denaturation. The liquid medium provided by the buffer maintains a constant adequate temperature during the run.
Meaning, that it reduces the temperature and prevents overheating.
Related article: 5 Interpretation Manifests RNA Degradation.
- Prevent electrolysis:
Electrical current often causes electrolysis– a decomposition process of a molecule. Buffer prevents electrolysis and maintains the integrity of nucleic acid during the run.
- Even current distribution:
The constant ionic strength provided by the buffer in the entire electrophoresis chamber helps distribute a constant and even current during the run. This is particularly important for the migration of all the fragments evenly without any disturbance.
- Neutralize charges:
During the run, the electrical current breaks down the water molecules into H+ and OH-. Both these ions migrate to their opposite ends and alter the pH. The buffer provides charged ions that neutralize water charges and protect our nucleic acid.
Thus, charge neutralization by buffer helps protect the integrity of the nucleic acid.
- Prevent nucleic acid degradation:
One of the pivotal applications of a buffer during electrophoresis is to deactivate any nucleases either DNase or RNase present in the sample. EDTA present in the buffer does this job.
As we also know the EDTA is a chelating agent. A nuclease requires a cofactor, for example– Mg+ ion for catalytic reaction. It degrades DNA, if everything will remain in its favor. Fortunately, the EDTA chelates the Mg+ ion, blocks the cofactor binding site and disables the nuclease.
Again this will protect the DNA or RNA and maintain their integrity.
In conclusion, a buffer maintains the pH, protects the nucleic acid, prevents hydrolysis and electrolysis, evenly distributes the current and neutralizes the charges.
Running Buffers used in electrophoresis:
Here is the list of the buffers that are commonly used in various types of electrophoresis techniques.
| Sr No | Buffer Name | Composition |
| 1 | TAE | Tris-acetate-EDTA |
| 2 | TBE | Tris-Borate-EDTA |
| 3 | MOPS 3-(N-Morpholino)-Propanesulphonic acid | MOPS, sodium hydroxide, sodium acetate and EDTA. |
| 4 | ACES | N-(2-acetamido)-2- aminoethaneulphonic acid |
| 5 | CAPS buffer | (N-cyclohexyl-3-aminopropanesulfonic acid) |
| 6 | Tricine buffer | Tris HCl, Glycerol, SDS, Commasie blue, |
Electrophoresis Techniques and Running Buffer Types:
| Electrophoresis technique | Buffer Type |
| Agarose gel electrophoresis | TAE and TBE |
| Polyacrylamide gel electrophoresis (PAGE) | Tris-Glycine and Tris-Tricin with SDS. |
| SDS-PAGE | Tris-Glycine and Tris-Tricin with SDS. |
| Capillary gel electrophoresis | Tris-Borate, Tris-Phosphate and ACES |
| Denaturing Gradient Gel Electrophoresis | TAE and TBE |
| Pulse-field gel electrophoresis | TBE only |
| Isoelectric focusing | Ampholyte buffer |
| RNA gel electrophoresis | MOPS (formaldehyde) |
Running Buffers used in various techniques:
| Technique | Electrophoresis buffer |
| Genomic DNA isolation | TAE |
| Plasmid DNA | TAE and TBE |
| PCR product | TAE for larger and TBE for smaller PCR products. |
| Restriction digestion | TBE |
| Southern blotting | TBE |
| DNA sequencing | MOPS and TBE |
| Cloning | TAE |
Related article: Common Issues in Gel Electrophoresis and Troubleshootings.
My Ultimate Guide to Using the Running Buffer:
I have years of experience, working in a molecular genetic laboratory. I ran hundreds of electrophoresis and analyzed thousands of samples. From my years of experience, I noticed a few important things that help in better electrophoresis.
Now you may wonder why it’s so crucial.
Let me tell you that when you deal with a diagnostic sample, you have to be careful. The results should be crystal clear and the interpretation should be 100% confident. However, the same is also applied to gene therapy and genetic engineering experiments too.
So you have to deliver amazing results, every single day in your lab. To do so, you have to take care of these things.
Freshly prepared reagents will always give you amazing results. Always prefer to prepare a fresh buffer for a day or two. As the buffer ages, it might become contaminated, some ingredients could lose their strength or it may lose its buffering capacity.
In addition, salts in the running buffer may dissociate over time. So try preparing important solutions, like the buffer early in the morning, first.
The second thing is, that it is better to check the pH of the working and even the stock buffer before use. This will save time, reagents and money.
Re-usability is always an issue in this case. You may wonder, can we reuse the buffer? The answer again is ‘It depends.’ You can reuse a buffer two times or even three times.
But not for the main or critical results. If you are doing some school or master’s level research, it’s fine! Go for it. But let me tell you that you will observe a substantial difference in the results.
Avoid reusing buffers so many times. Because repeated use decreases the ionic and buffering strength.
Use the same batch of buffer for gel preparation and electrophoresis running. For example, suppose you have prepared two stock solution batches– Buffer A and Buffer B. If you use buffer A for gel preparation use the same for electrophoresis run.
Using buffers from two different batches can negatively impact the quality of the results.
Check the quality of a running buffer periodically. Observe the color change, crystallization, and visible contamination. If these occur, discard the buffer and prepare a fresh new batch.
Store a working and stock buffer in a fresh and sterile container.
Always prepare a buffer using distilled water only.
Read more: 10 Proven Tips to Success In Gel Electrophoresis of DNA.
Wrapping up:
A running buffer plays a crucial role during the gel electrophoresis. And it’s one of the factors that influence the results. TAE and TBE are the two most common buffer types used in various types of electrophoresis. Hope your electrophoresis buffer concepts are now clear and I’m confident that you can prepare and use it effectively.
I have prepared a dedicated article on the TAE and TBE, you can read it, the link is given at the start of this article. That’s it for this topic. You can use the search box to read other related articles.
Sources: The 9 Best Biological Buffers for Electrophoresis by HOPAX.


