“TAE and TBE are the two most common and popular buffer systems used in the gel electrophoresis of DNA, RNA and proteins. Explore some differences and conditions to use it.”
A biological buffer is used in the reaction to maintain the pH but it has other crucial roles as well. It is a combination of a weak acid and conjugate base and vice versa.
Various types of buffer systems are designed for various experiments, for example— TBE and TAE buffers for gel electrophoresis and TE buffer for DNA re-hydration.
Electrophoresis, in a nutshell, is a technique to separate molecules, like— DNA or RNA based on their electrical charges and weight. Thus, it helps to perform nucleic acid-based analysis. Electrophoresis is employed in PCR, DNA sequencing and restriction digestion-like experiments.
A buffer is a crucial component of any electrophoresis experiment having various important functions besides controlling the pH. In this article, I will compare and explain the two most common buffer systems used in molecular biology– TAE and TBE buffer.
We will comprehensively understand the composition, recipe and significance of both here. At last, I will provide my ultimate guide to choosing a buffer for your experiment.
This article is going to be a technical blueprint for your molecular genetic endeavor. Stay tuned.
Related articles:
- Different Types of DNA/RNA Gel Electrophoresis and Their Applications.
- Common Issues in DNA/RNA Gel Electrophoresis and Troubleshooting.
- Factor Affecting DNA Agarose Gel Electrophoresis Results.
Key Topics:
What is an electrophoresis buffer?
An electrophoresis buffer is also known as a running buffer or electrophoresis running buffer. Put simply, an electrophoresis buffer is a solution used during the agarose gel electrophoresis of either DNA or RNA. It usually consists of ingredients that
- Maintain the pH.
- Provide a liquid medium for migration.
- Provides ionic strength.
- Prevents nucleic acid from nuclease attack.
- Prevent hydrolysis of DNA or RNA.
In conclusion, it helps in the effective migration of nucleic acid in the electrophoresis which eventually gives us good resolution and results. Two common buffer systems, as aforementioned, are TAE (Tris-acetate-EDTA) and TBE (Tris-Borate-EDTA).
To know more, read this article: 7 Purposes of a Running Buffer in Electrophoresis + My Guide to Use It.
Let’s talk about each one, one by one.
TAE buffer:
Major constituents of TAE buffer are Tris base, Acetic acid (acetate) and EDTA (Ethylenediamine tetraacetic acid). Here, Tris maintains the pH, acetic acid enhances the electrophoretic mobility of nucleic acid and EDTA works as a chelating agent.
EDTA prevents DNA degradation. It prevents the cofactor binding for the enzyme and thus, makes the enzyme (nuclease) inactive.
Benefits:
- TAE can run larger DNA fragments effectively due to its better conductivity, however, the conductivity is lower than the TBE buffer. Studies suggest that for fragments larger than 2 Kb in size, the TAE buffer is the first choice.
- In addition, it provides a faster migration rate compared to the TBE buffer.
- It has a higher nucleic acid recovery rate compared to the TBE buffer.
- It’s cheaper.
- 0.5% TAE buffer can work effectively and thus, it comes up with a lower working solution requirement.
Due to these reasons, TAE is used for long-range PCR, genomic DNA separation, cloning and genetic engineering experiments.
Limitations:
- It has a lower buffering capacity. So has lower re-usability.
- It can not be used for the separation of smaller products like restriction digestion.
Recipe for 10X TAE buffer:
- 400mM Tris
- 200mM acetic acid
- 10mM EDTA
- Add D/W to make the final volume of 250ml
(10X) 250ml TAE buffer is our stock solution using which we can prepare our working solution 10 times.
Recipe for 1X TAE buffer:
To prepare a 1X TAE buffer, which is the working solution, we have to take 1 ml 10X TAE buffer (stock) into 9 ml distilled water. As we required approximately 1000 ml buffer for a typical electrophoresis run, add 100 ml 10X TAE buffer into 900 ml distilled water to make it 1X.
You can also use 0.5X TAE buffer as well.
How to prepare a TAE buffer?
- Add 10 mM EDTA into 400 mM Tris and 200 mM acetic acid.
- Mix it until the EDTA dissolves completely.
- Adjust the pH of the solution between 8.0 to 8.5.
- Make up the final volume up to 250 ml using the distilled water.
- This is the 10X working solution to prepare the 1X working solution, follow the process explained above.
TBE buffer:
Major constituents of the TBE buffer are Tris, Borate or boric acid and EDTA. Here, instead of acetic acid, boric acid is used. Tris and boric acid both provide stable pH as well as maintain it. While the EDTA works as a chelating agent.
Benefits:
- TBE buffer has a higher buffering capacity than the TAE buffer due to the presence of borate or boric acid.
- It’s ideally recommended for DNA fragments < 1500 bp.
- Borate inhibits many enzymes including the nucleases and thus, DNA remains in highly intact form during the run.
- It is used in native gel agarose gel electrophoresis, PAGE, Pulse-field gel electrophoresis and DNA sequencing.
- It also works as an alternative to the TAE buffer.
Limitations:
- TBE buffer has a slower migration rate.
- It has a poor resolution for longer DNA fragments.
- The nucleic acid recovery rate is very low as borate may interact with the DNA.
- It requires a 1X working solution which is comparatively higher than the TAE buffer.
- It is ideally not recommended for cloning and genetic engineering experiments.
In conclusion, the TBE buffer can also be used as an alternative to the TAE buffer for selective conditions.
Recipe for 10X TBE buffer:
- 90mM Tris
- 89mM Borate
- 2mM EDTA
- Make up the final volume of 250 ml using distilled water.
Recipe for 1X TBE buffer:
The recipe for 1X working TBE buffer will remain the same as we prepared the 1X TAE buffer. Add 1 ml of 10X TBE buffer into 9 ml of distilled water to make 1X TBE buffer.
Calculate for 1000 ml accordingly.
How to prepare TBE buffer
- Add 2mM EDTA into 90mM Tris and 89 mM Borate.
- Mix well until the EDTA dissolves completely.
- Adjust the pH between 8 to 8.5.
- Prepare 1X working solution as discussed above.
Read more: Clash of Gels- Agarose Gel vs PAGE Gel.
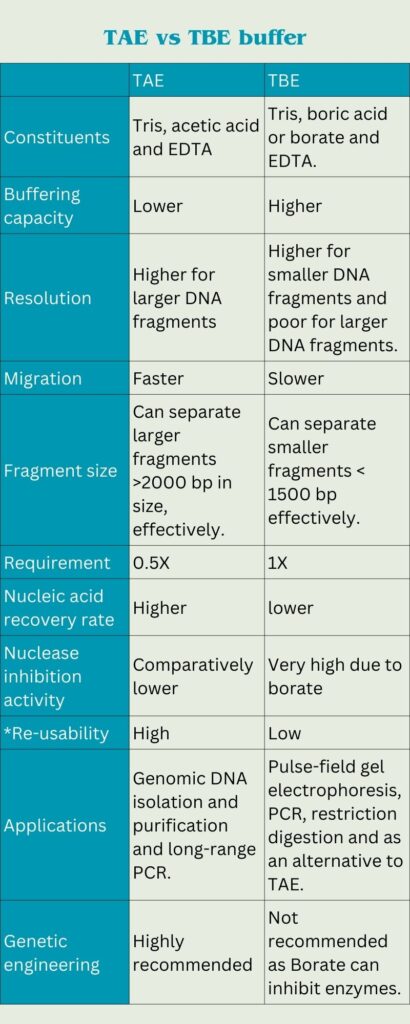
Differences between TAE and TBE buffer:
| TAE | TBE | |
| Constituents | Tris, acetic acid and EDTA | Tris, boric acid or borate and EDTA. |
| Buffering capacity | Lower | Higher |
| Resolution | Higher for larger DNA fragments | Higher for smaller DNA fragments and poor for larger DNA fragments. |
| Migration | Faster | Slower |
| Fragment size | Can separate larger fragments >2000 bp in size, effectively. | Can separate smaller fragments < 1500 bp effectively. |
| Requirement | 0.5X | 1X |
| Nucleic acid recovery rate | Higher | lower |
| Nuclease inhibition activity | Comparatively lower | Very high due to borate |
| *Re-usability | High | Low |
| Applications | Genomic DNA isolation and purification and long-range PCR. | Pulse-field gel electrophoresis, PCR, restriction digestion and as an alternative to TAE. |
| Genetic engineering | Highly recommended | Not recommended as Borate can inhibit enzymes. |
*avoid re-using the buffer if you don’t have the expertise to use it. Take an expert’s help. Re-use can degrade or deteriorate results.
How to choose an electrophoresis buffer?
Now, things are a bit confusing! You may wonder which buffer to use. And when? For the selection of any chemical or solution, the common answer would– depends on your experiment!
Let me explain it in detail.
For example, if you are doing some random experiments or non-crucial experiments (just a school-level thing!) you can use either buffer. Both can work. However, you have to worry a bit, when your experiment is so important.
For example– diagnosis, genetic engineering and some crucial research projects. In such conditions, you have to carefully select the buffer. Let me explain some scenarios.
TAE buffer is used in these conditions.
Routine experiments and educational explanation: TAE is a cost-effective option and thus used routines for the separation of larger and smaller DNA fragments and for some school-level experiments.
It is also widely used during DNA extraction and purification for validation as it can separate supercoiled DNA more effectively.
In addition, its particular application is in the separation of larger DNA fragments. So whenever the experiment deals with larger fragments >2000 bp, one must have to choose the TAE buffer.
It is also particularly used for certain applications having specific enzymes involved. For example, cloning or recombinant DNA techniques, etc.
TBE buffer is used in these conditions.
You can use TBE where you have to deal with the smaller-sized fragments, for example, PCR analysis or restriction digestion.
You can use TBE for high-resolution electrophoresis analysis for sharpen banding patterns.
TBE is an ideal choice for DNA sequencing, nucleic acid size determination and RNA electrophoresis.
Read more: How to Extract DNA From a Gel?- A Step-By-Step Guide.
Wrapping up:
In conclusion, you can use the TAE buffer without any doubt for any experiments, however, to get amazing and a bit more sharp results (for some particular conditions that I have mentioned) you can use the TBE buffer.
Either buffer plays a pivotal role in electrophoresis. However, various concentrations, combinations and types of buffers are now commercially available. So you do not need to deal with a tedious solution preparation procedure.
I hope you like this article. Please share it in your study circle.
P.S. Check out our PCR mastery course.
Sources:
Sanderson BA, Araki N, Lilley JL, Guerrero G, Lewis LK. Modification of gel architecture and TBE/TAE buffer composition to minimize heating during agarose gel electrophoresis. Anal Biochem. 2014 Jun 1;454:44-52. doi: 10.1016/j.ab.2014.03.003.


Hi Sir kindly share Hb and serum proteins Electrophoresis