“Topoisomerase is a class of enzymes that helps in winding and unwinding of DNA. Three forms of DNA are most prevalent in nature: circular, linear and supercoiled.”
Circular DNA is covalently closed and does not have any interruption between nucleotides. Circular DNA is found in cytoplasmic organelles and bacteria.
Linear DNA molecules have free ends on both sides, hence linear DNA has free 5’-P and free 3’-OH groups present on two opposite ends. Generally, linear DNA is found in some bacteria and cytoplasmic DNA in some algae.
Supercoiled DNA is made up of twists and writhes. Eukaryotic DNA is supercoiled and helps in the packaging of DNA on chromosomes.
Read more about DNA packaging in eukaryotes
Simply put, when DNA undergoes additional twisting and coiling on each other, it is called supercoiling. As eukaryotic DNA is longer and contains more base pairs, it is important to develop a supercoiled form.
Supercoiling gives a compact arrangement to DNA which is necessary to place it in the nucleus, but supercoiling has one drawback. It hinders replication and transcription.
Recall the process of replication, DNA helicase unwinds the double strand of DNA and increases tension on an unwinded double strand of DNA. DNA topoisomerase helps in removing tension from remaining unreplicated DNA.
Two types of DNA topoisomerase enzymes are prevalently found depending upon their function. Both perform different functions during different stages of coiling and supercoiling.
Related article: DNA polymerase.
Key Topics:
DNA topoisomerase-I
Double-stranded DNA is arranged in a helix. This spiral arrangement of dsDNA increases tension inside the double-strand which tightens the double helix. DNA topoisomerase-I works in the unwinding of double-stranded DNA. Here topoisomerase-I cuts one strand of DNA and allows another strand to pass through cut and rejoins the end.
After completion of each topoisomerase-I activity, double-stranded DNA unwinds and linking the number of DNA is changed in a single step. Additionally, topoI is ATP independent, which means it does not require ATP as an energy source for completing its function.
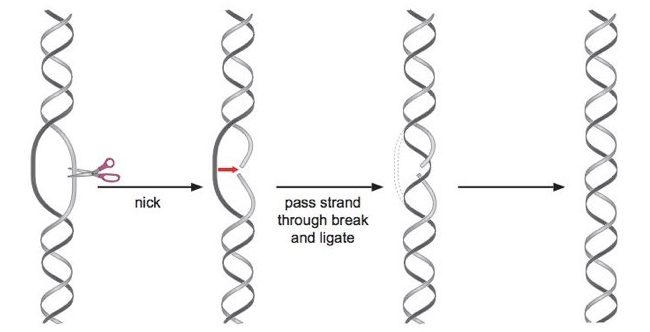
DNA topoI is subdivided into topo IA and topo IB. Topo IA is usually bacterial topoisomerase whereas topo IB is eukaryotic topoisomerase. Prokaryotic DNA topoisomerase can only relax negatively supercoiled DNA, in contrast, eukaryotic topoisomerase can relax positively supercoiled DNA and replicating DNA.
Read the article: DNA Replication: The General process of DNA replication
DNA topoisomerase II
DNA topoisomerase II is ATP dependent enzyme that required 2 ATP molecules per reaction. It acts of entire double-stranded DNA, cuts it and rejoins it. Topo II relaxes positive supercoiling in eukaryotic DNA.
One special type of DNA topoisomerase II found in prokaryotes is named “DNA gyrase” which introduces supercoiling in bacterial DNA. DNA gyrase performs both functions of releasing as well as introducing negative supercoiling in bacterial DNA.
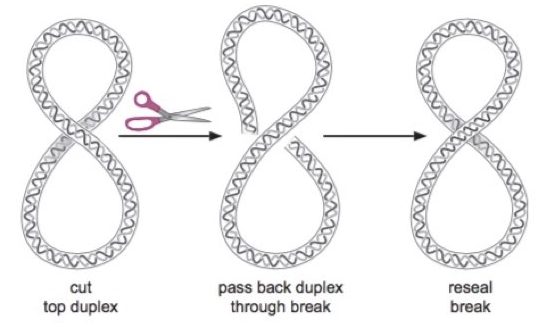
Another important function performed by topoII, are catenation and de-catenation. Imagine two linked rings. DNA molecules are catenated in the same manner. Here, topoisomerase cut the dsDNA and decatenate it for relaxation. Further, it catenates the decatenated DNA for supercoiling.
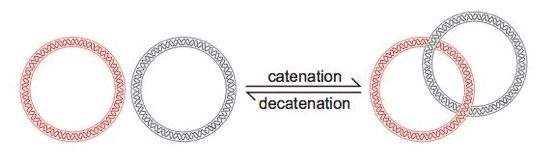
The process of decatenation is a very important process as it allows the separation of the DNA molecule into two daughter cells, after replication.
Mechanism of action:
Topoisomerase cleaves and ligates DNA in a single reaction and without the use of any energy. But for the completion of any biological reaction, energy must require. Then how does topoisomerase perform this function without any energy?
Here topoisomerases perform covalent intermediate interaction mechanisms. Topoisomerase interacts as an intermediate between two ends of the broken DNA strand. When a DNA molecule is broken, the phosphodiester bond between the DNA molecule is also broken.
The tyrosine residue of activated topoisomerase attacks directly the phosphodiester bonds of broken DNA. The tyrosine is now bound with the phosphate of the broken DNA strand at the -5′ end. The other -3′ end remains free and held by the topoisomerase domain.
The interaction between tyrosine of active topoisomerase and phosphate of DNA is weak covalent and it conserves energy for rejoining strands. Another strand (in case of topoisomerase I) or another double helix (in case of topoisomerase II) passed through the broken end.
Here the free -OH group destroys the weak intermediate covalent bond between phosphate and tyrosine (of topoisomerase) and rejoined with phosphate by a phosphodiester bond.
Topoisomerase is released from the site of action and moves to another site for performing another reaction. Importantly, an energy molecule is not utilized by any topoisomerase.
Then what happens with the ATP molecule which is consumed by topoisomerase-II? The energy of ATP hydrolysis is used to promote conformational changes in the topoisomerase-DNA complex, not for cleaving and ligating.
Enzyme open bridge
Conformational changes in the shape of the enzyme are very important for relaxing DNA molecules. here the energy from ATP hydrolysis (in the case of topo II) is utilized to perform this function.
In the first step, the enzyme recognizes the DNA molecule as a substrate and binds to it. More specifically, its affinity is higher in the case of supercoiled DNA.
Now in the second step, tyrosine-dependent activity leads to creating a gap between a DNA strand and covalently binds to phosphate. Here the enzyme opens both the end by making the conformational change in its shape and creates a bridge for another strand to pass through it.
In the next step, the intact DNA strand is passed through it and one special domain of topoisomerase held the DNA strand until the bridge is closed and the broken DNA stand is joined.
Read more:
- Meet DNA Primase: The Initiator Of DNA Replication.
- “Transcription And Translation” A Brief Overview.
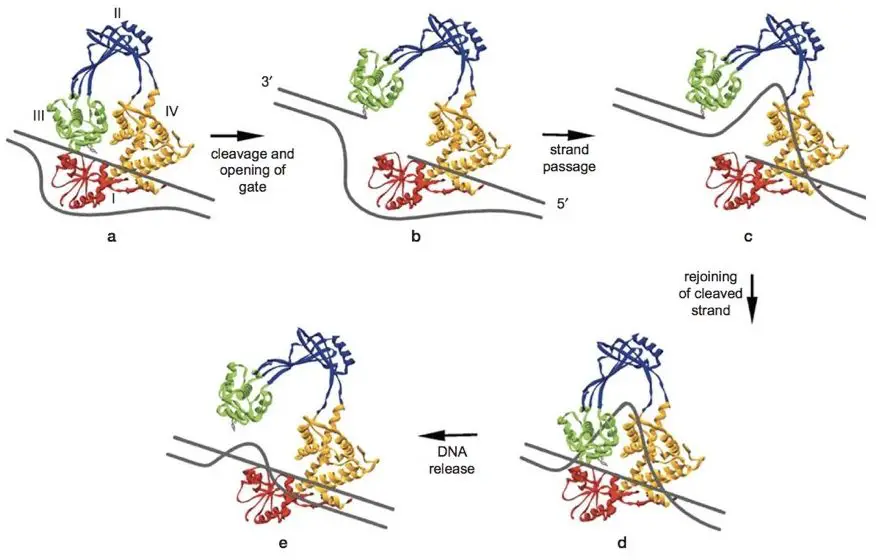
At last one final time, the enzyme opens up and releases the active site and moves to another site. The mechanism is similar in both types of topoisomerase. But topoisomerase-I passes a single strand and topoisomerase-II passes double-stranded DNA.
Additionally, topoisomerase-II is dimeric or tetrameric because it has to work on breaking and ligating both strands of DNA. Energy (in the form of ATP) is required for making conformational changes in these extra domains.
Topoisomerase maintains the speed of replication by unwinding DNA and releasing tension. Supercoiled DNA has twists and writhes which makes it complex. We will discuss twists, writhes, cccDNA, and linking numbers in the next article.
Conclusion:
The topoisomerase is as important as other enzymes involved in DNA replication. If not there, the process of replication can’t be completed. This will cause a replication error.
Replication is necessary for us to survive and grow.


Very awesomely described. Thank You
if you like our article than please subscribe to our blog.