“Understand the concept of gene amplification, its significance and mechanisms and learn how it is involved in cancer and how to detect it.”
Amplification is a popular concept in the PCR, where a DNA fragment is amplified, meaning, ‘copied.’ Let me introduce to you the actual concept of gene amplification in the natural system.
Amplification helps some organisms as a part of their development and defense system, while in others, it can cause cancer.
For instance, it has a significant role in embryonic development in amphibians, and at the same time, the same mechanism causes cancer in humans. Chromosomal translocations, gene duplications, gene fusions and gene amplifications are commonly observed in cancer.
Sounds interesting!
In this article, we will introduce gene amplification, discuss various mechanisms and the techniques that we can use to study and investigate it. And its role in cancer, obviously.
Stay tuned.
Disclaimer: The content presented herein has been compiled from reputable, peer-reviewed sources and is presented in an easy to understand manner for better comprehension. A complete list of sources is provided after the article for reference.
Key Topics:
What is Gene amplification?
Genes are present in copies, each on each homologous chromosome. The amount in which a particular gene expresses and produces the protein is a ‘matter of concern’ for a cell. Hence, depending on the requirement, cells express genes differently.
This process is cell or tissue-specific and highly regulated. Unfortunately, over or under-expression causes imbalance and abnormality. Gene amplification is when a gene crosses the boundaries of its gene expression level and over-expresses.
Keep in mind that the term gene amplification is applied to the event that increases the copy number of a gene or any DNA sequence in a haploid genome over or above the normal value.
However, in normal circumstances, it is a regulated process. Studies reveal gene amplification as a positive regulation in some organisms, including prokaryotes and during the developmental stages.
Definition:
Gene amplification is defined as a mechanism of increasing gene copies in a genome or chromosomal regions over the normal. Uncontrolled gene amplification causes cancer.
Gene amplification in normal conditions:
The present phenomenon is a crucial event that occurs in cancer. However, it is further reported in several organisms to fulfil their developmental and protein synthesis needs and defense against environmental factors.
Thus, the significant role of gene amplification has been reported in certain amphibians and drosophila oocyte developmental stages.
Gene amplification in amphibian oocytes:
Bostock (1986) explained the importance of gene amplification in amphibian gametogenesis. The ribosomal RNA gene- one of the structural genes located tandemly into the genome- is a classic example of gene amplification in amphibians.
The present study showed that during the normal somatic cell division, it replicates in sufficient amounts to meet the cell’s protein requirement, but not in the gametogenesis.
In the oocytes, a large amount of ribosomal proteins is needed to support protein synthesis. Hence, the rRNA gene amplifies exponentially to meet the cell’s protein requirement.
rRNA gene amplification has been reported starting from 40-fold to as high as 250-fold. Notedly, the higher need could not be completed by natural replication, rather, it amplifies using an alternative rolling circle mechanism, multiple times, in the same cell.
This ensures that the developing embryo will get a sufficiently higher amount of the ribosomal RNA gene for rapid growth and differentiation.
Gene amplification in drosophila:
Stormo & Fox (2018) explained localized gene amplification in the drosophila polytene chromosome for higher requirements of digestive enzymes and structural proteins. Polytene is the largest chromosome present in many organisms.
It increases in size by the process known as endometriosis or endocycle. This is a process of replication without cell division. The higher protein requirement during the larval development stage causes gene amplification on a polytene chromosome, particularly in the salivary gland.
Gene amplification occurs from 1,000-fold to 1,000,000-fold, depending on the tissue and its requirement. This makes the size of the polytene chromosome nearly ~10mm in drosophila.
Gene amplification in bacteria:
Another classic and well-studied example of gene amplification is antibiotic resistance gene amplification in bacteria. The recent paper published by Silva and Khare (2024) explains the implication of gene amplification against antibiotics in healthcare.
When bacteria encounter an antibiotic, the antibiotic resistance gene tandemly amplifies and rapidly increases the gene expression in the bacterial population. By doing so, it neutralizes or expels the effect of the antibiotics.
Notedly, bacteria use diverse mechanisms for gene amplification as per the requirement and antibiotic used. Rapid antibiotic resistance is a common problem in infection treatment and can also lead to multidrug resistance in several conditions.
That’s amazing; these organisms and many others, excluding humans, have a pre-built mechanism of gene amplification. Using this, they can counter-regulate their protein requirements during different stages of their life.
However, gene amplification is a curse for us!
Sudden or unwanted gene amplification in human genes results in a catastrophe called cancer.
Let’s understand how gene amplification contributes to cancer and several well-studied mechanisms that contribute to the situation.
Gene Amplification and Cancer:
In our beginner’s guide to cancer genetics, I explained the genetic factors behind cancer. Let me just give you a brief understanding.
Proto-oncogenes and tumor suppressor genes regulate the cell cycle and cell division process. Proto-oncogenes promote cell growth while tumor suppressor genes regulate or reduce cell division and guide cells to apoptosis.
The whole cell cycle and division process are highly regulated by these two classes of genes. Imbalance causes infinite cell division, escapes apoptosis and leads to cancer.
The gain of function mutations in proto-oncogenes and loss of function mutations in tumor suppressor genes slip the control over cell division and result in cancer.
A gain-of-function mutation means the gene copies increase abnormally, followed by an increment in protein synthesis.
What does that mean?
That’s gene amplification! Proto-oncogene-mediated tumorigenesis is caused by gene amplification.
The mutant counterparts of the proto-oncogenes, the oncogenes, once formed, undergo gene amplification. This will increase the abnormal oncogene copies above the limit, imbalance the mechanism and promote tumor formation.
In addition, the continuous high oncogene amplification also helps the tumor spread to other tissues and distant organisms. Check out the table below to know more about common genes, their amplification rate and their association with cancer.
| Gene | Cancer | Amplification rate |
| HER2 | Breast Cancer | ~20-30% |
| MYC | Neuroblastoma, Lung, Breast Cancer | ~40% |
| EGFR | Glioblastoma, Lung Cancer | ~50% |
| MDM2 | Sarcomas, Gliomas | ~10-20% |
| CCND1 | Head & Neck, Breast Cancer | ~15-20% |
| CDK4 | Melanoma, Sarcomas | ~10-15% |
Here, I am explaining various well-researched gene amplification mechanisms in cancer.
Mechanisms causing gene amplification
Replication copies genes in a natural process and for a cell. However, it’s a very tightly controlled process and only occurs during cell division. It copies genes only if the cell requires it.
In gene amplification, genes replicate autonomously in the cell and without cell division. This exponentially increases the amplified gene copies. Check out several mechanisms for gene amplification.
Related article: Proto-oncogenes vs Tumor Suppressor Genes- Key Differences.
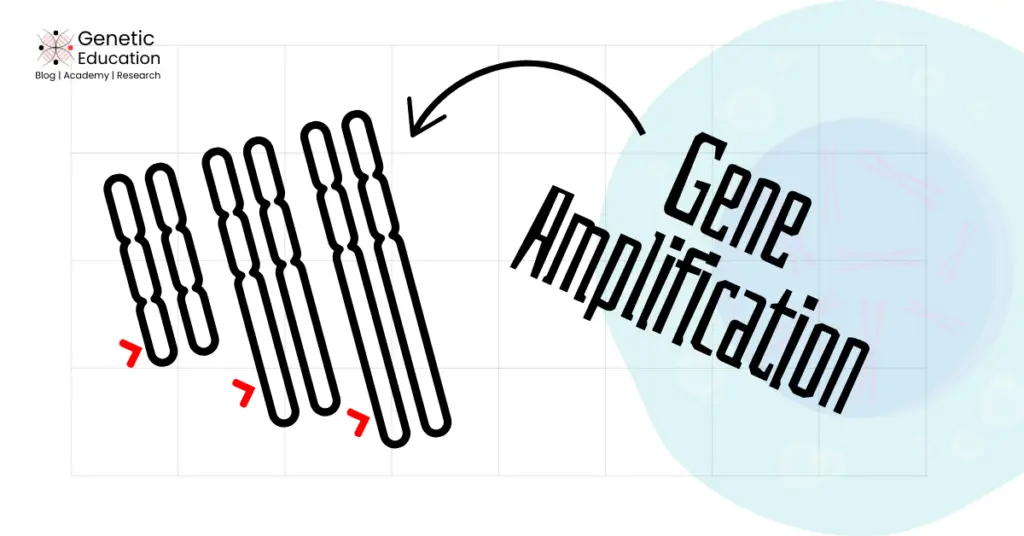
Double-minute chromosomes:
Double minutes have been extensively reported in many human cancers but are most common in brain cancer and neuroblastoma. It is an extrachromosomal DNA or chromatin commonly present in tumor cells.
The DM forms due to the amplification of proto-oncogene-rich chromosome regions and appears as circular and extrachromosomal structures. However, it is not a true chromosome as it lacks centromere and telomeres.
It replicates autonomously within the nucleus, can not segregate during cell division and produces excess oncogene copies. Thus, a few mega base pair structures appear as double-stranded dots under the microscope and hence the name is given.
The exact mechanism of DMs isn’t yet understood. Several adverse conditions cause chromosomal rearrangements, however, some DNA can’t re-integrate into the genome and appear as double minutes.
It circularized and became an active DM. Double minutes are commonly observed in neuroblastoma and glioblastoma and for the genes MYC and EGFR.
Studies showed approximately 1.4% prevalence of DM in various cancers and 31.7% in neuroblastoma. Noteworthy that gene amplification by the higher double-minute chromosome poses additional challenges in treatment.
Homogeneously staining regions:
HSRs amplify oncogenes tandemly by forming a continuous amplification gene block. It is mostly similar to the double minute chromosome, but unlike DM, it remains attached to the chromosome.
It appears in a uniformly or homogeneously stained chromosomal region under the microscope, thus, the name has been given. Commonly observed in breast, lung and colorectal cancer.
Rolling circle amplification
Rolling circle replication is also reported in amphibian gene amplification. In cancer, it is the next step after the double minute chromosome. Now, what happens here is once the oncogene DNA is circularized and becomes extrachromosomal, it replicates on its own.
Using the mechanism known as rolling circular amplification, it amplifies the gene copies and makes them more distinct and visible as DMs or HSRs. MYC and EGFR-like oncogenes amplify through the rolling circle model.
Breakage-fusion-bridge cycle:
BFB cycle is significant in tumors with high chromosomal instability. Here, chromosomes first experience random double-stranded breaks. The failed DNA repair pathway in the tumor can’t repair the break.
Consequently, it is fused abnormally to any chromosomal location, forms a bridge between sister chromatids and breaks abnormally. The process continues with consecutive cell divisions.
In this never-ending process, oncogene amplification leads to tumor progression and spread.
How does Gene amplification cause cancer?
Here is the complete stepwise process of how gene amplification causes cancer.
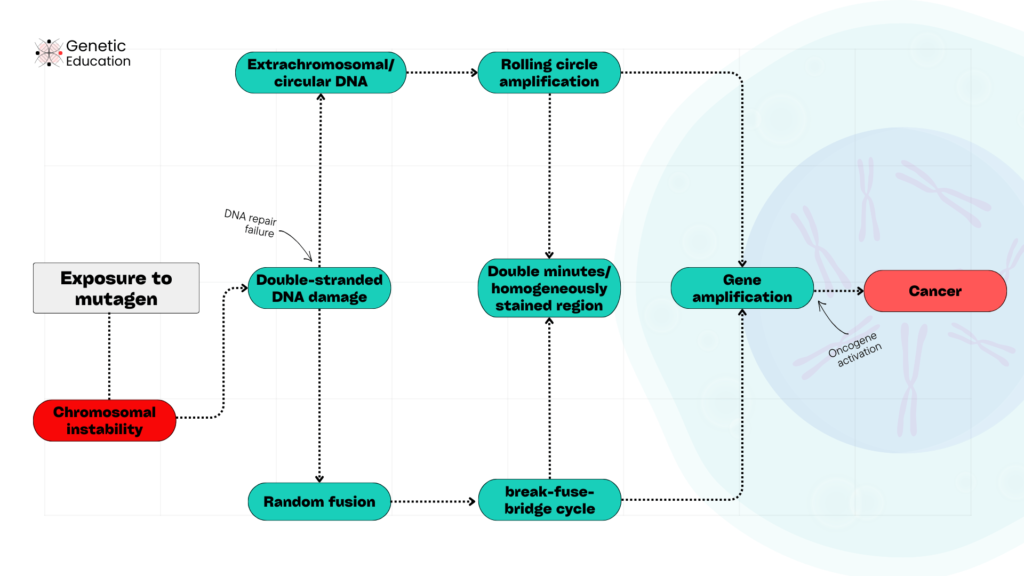
- Exposure to mutagens causes double-stranded DNA breaks at many random sites on chromosomes and produces chromosomal instability.
- Common mutagens are radiation, carcinogens, viral infection, pollution and replication errors.
- Under excessive pressure, the DNA repair mechanism fails to repair the errors.
- The double-stranded breaks either fuse randomly to chromosomal regions or circularize and become extracellular DNA.
- The random fuses follow the breakage-fusion-bridge cycle, while the circular DNA follows rolling circle amplification.
- In both cases, proto-oncogene counterparts, the oncogene amplifies in higher amounts.
- If it is a rolling circle, then it appears as either a double minute chromosome or a homogeneously stained region.
- Amplified oncogenes imbalance the cell division, escape the apoptosis and trigger damaged cell division, and form tumors.
Detection of gene amplification:
Now, gene amplification can be detected using various techniques. Karyotyping and FISH are the classic techniques to investigate double minutes and homogeneously stained regions.
Despite having limited resolution, karyotype can identify chromosomal instabilities reported in the article. Abnormal karyotype with double minutes, HSR, breakage fusion gene amplification, etc. can be detected using GTG, reverse and fluorochrome banding.
However, a better resolution can be achieved using fluorescence in situ hybridization. FISH allows us to study targeted gene amplification from the whole karyotype.
Both techniques have been considered a gold-standard and routine method for gene amplification and other chromosomal instability studies. However, experience and expertise are required.
We explained both the techniques in our previous article and course on karyotyping and FISH. You can check out the free resources or courses to learn more.
The new-age genetic technologies are now extensively used to study gene amplification and quantify the amount.
Techniques like quantitative PCR, RNA and gene expression microarray are instrumental in determining the quantitative analysis of gene amplification. Where PCR and DNA sequencing provide sequencing level information regarding the gain-of-function mutations, amplification region, etc.
Futuristic techniques like Next-generation sequencing or single molecular real-time sequencing have been employed for high throughput cancer or gene amplification studies and analysis.
They are capable enough to investigate many gene amplification regions in a single experiment. Note that the chromosomal microarray is also a high throughput technique used for chromosomal instability studies.
Wrapping up:
In conclusion, gene amplification is beneficial to several organisms but not to humans. It is an important genetic marker in cancer studies and research. Amplification of oncogenes induces tumorigenesis and spread.
It is still unknown to us how other organisms used this catastrophic and lethal event for their benefit. Are they superior to us?
God knows!
I hope you like this article, share it and subscribe to Genetic Education.
Sources:
Bostock CJ. Mechanisms of DNA sequence amplification and their evolutionary consequences. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 1986;312(1154):261-273. doi:https://doi.org/10.1098/rstb.1986.0006.
Stormo BM, Fox DT. Polyteny: still a giant player in chromosome research. Chromosome Research. 2017;25(3-4):201-214. doi:https://doi.org/10.1007/s10577-017-9562-z
Silva, Khare A. Antibiotic resistance mediated by gene amplifications. npj Antimicrobials and Resistance. 2024;2(1). doi:https://doi.org/10.1038/s44259-024-00052-5.
Xu K, Ding L, Chang TC, et al. Structure and evolution of double minutes in diagnosis and relapse brain tumors. 2019;137(1):123-137. doi:https://doi.org/10.1007/s00401-018-1912-1
Frater JL, Hoover RG, Bernreuter K, Batanian JR. Deletion of MYC and presence of double minutes with MYC amplification in a morphologic acute promyelocytic leukemia–like case lacking RARA rearrangement: could early exclusion of double-minute chromosomes be a prognostic factor? Cancer Genetics and Cytogenetics. 2006;166(2):139-145 (Image).
Taguchi T, Kubota S, Mezaki T, et al. Identification of homogeneously staining regions by G-banding and chromosome microdissection, and FISH marker selection using human Alu sequence primers in a scleractinian coral Coelastrea aspera Verrill, 1866 (Cnidaria). Comparative Cytogenetics. 2016;10(1):61-75 (Image).
Attractive section of content I just stumbled upon your blog and in accession capital to assert that I get actually enjoyed account your blog posts Anyway I will be subscribing to your augment and even I achievement you access consistently fast
This was such a well-researched and insightful piece.
Your post is not only informative but also incredibly well-structured. I really appreciate the logical flow from one point to the next, and the examples you’ve included have helped to solidify the key concepts. This is a post I’ll be sharing with others who will find it equally valuable.