“Maternal cell contamination is defined as the presence of maternal cells in a fetal sample such as amniotic fluid or chorionic villi. It creates preanalytical problems during testing.”
Prenatal genetic tests have a significant role in the management and prevention of many genetic disorders. It precisely dictates the likelihood of the fetus’ risk for getting a genetic condition.
It can predict the condition (either normal, homozygous or heterozygous) and prevent the spread of several diseases like down syndrome and thalassemia, however, is restricted to a few types of genetic disorders. It is especially used for the screening/diagnosis of monogenic conditions.
Prenatal testing is a specialized technique that requires special permissions to perform and so one should have experience and expertise to handle prenatal samples, as are precious and limited.
I was in a prenatal research program during my Ph.D. and learned various techniques and methods. One such ‘tool’, I can say, is the MCC – maternal cell contamination testing which has been acknowledged as an important parameter for prenatal genetic testing.
During the tenure, I have taught handling of prenatal samples, purification and washing techniques, DNA extraction methods for that and optimization techniques to deal with low quantity samples.
I have been writing blogs for 4 years and now I realize to write on this topic because when I searched the term, I literally found nothing, except the list of tests only. So I decided to write on; and share my knowledge, what I know about, in this blog.
In the present article, I will make you understand what maternal cell contamination is and how it is detected. I will also try to explain what the result looks like.
Stay tuned.
Related article: Cell-Free DNA Test- What is it & How to do it?
| Test type | Prenatal genetic testing |
| Objective | Detection of maternal cell contamination |
| Samples required | Maternal and paternal blood- 5ml; Fetus sample AF- 10 to 15ml; CV- a few visible CV tissues |
| Testing time | 10-20 days |
Key Topics:
What is maternal cell contamination?
Maternal cell contamination is the presence of maternal blood/cells or DNA in the fetal sample.
Prenatal genetic testing– testing of fetus relies on two types of samples viz amniotic fluid and chorionic villi tissues, collected directly from the fetus of a pregnant woman.
Either type of sample is rich in fetal tissues or cells and is sufficient enough to perform various genetic tests. Cells can directly be used for molecular analysis or karyotyping by DNA extraction or cell culture, respectively.
Notedly, testing procedures like PCR, sequencing or microarray remain the same as used in other testing but sample preparation and handling require special attention.
Chromosomal disorders like trisomies or aneuploidies (like down syndrome, Patau syndrome or Klinefelter syndrome) and single-gene disorders like thalassemia, sickle cell anemia and others can be diagnosed accurately by prenatal genetic testing.
Techniques like conventional karyotyping, FISH, microarray, PCR and DNA sequencing are utilized for this purpose. However, the presence of maternal cells in the sample restricts the testing and creates preanalytic problems.
Lamb et al., 2012 explained in their paper that standard cytogenetic analysis such as karyotyping, the MCC is less problematic as it can’t grow on the selective fetal media. Henceforth there are rare chances to interfere with fetal karyotyping. Notwithstanding, it is a definite problem for molecular analysis.
So the presence of maternal cell contamination results in a test failure, restricts preanalytical interpretation and misleading results.
Amniocentesis leads to miscarriage, sometimes and can’t be repeated hence we, as a researcher have only a single chance to process the sample. In case of failure, we can’t ask for repeat sampling.
We will explain each scenario for each type of sample separately.
Maternal cell contamination in amniotic fluid:
The amniotic fluid sample type is less prone to maternal contamination (compared to CV). Amniocentesis often causes fetal loss, if not performed correctly. AF is collected from the amniotic sac by using a long-sharp needle.
To possibly avoid accidental maternal cells, a few ml samples along with the syringe can be discarded. Studies depict that every 50 AF samples out of 100 contain more or less maternal blood cells.
A red-looking AF is a clear sign of MCC. As aforementioned, we can’t order a repeat collection, we must have to process the sample. There are several ways using which the MCC can be reduced or removed completely, called washing procedures.
For amniotic fluid, first, the cells are palletized by centrifugation following the removal of extra fluid. Keep the fluid stored, it can be reused; second, examine the pellets carefully to identify the blood cells.
Make sure to centrifuge the sample gently.
As per my experience, even if it shows clear pellets, it should contain maternal blood cells. Henceforth, the pellet must be processed through washing.
Washing is performed by adding TE buffer to the pellet followed by centrifugation. Wash twice or thrice with TE buffer until visibly clear pellets appear. The final sample is sent for DNA extraction.
Maternal cell contamination in chorionic villi:
Yet another common sample type of prenatal testing is a chorionic villus, abbreviated as CV, which is a solid tissue collected from the fetus. In comparison to amniotic fluid, studies describe that chorionic villi sampling is more prone to MCC.
A CV tissue sample should be clear, white and un-bloody. The presence of maternal decidua or blood spots indicates contamination. Studies relieved that CV contains more than 50% of MCs (Nuss et al., 1994). These types of samples must be washed first to remove blood decidua, before testing.
The CV sample washing is a comparatively tedious and time-consuming process, as is performed manually.
The chorionic villi sample, collected in the sterile container is emptied into the petri dish. TE buffer is added up to several marks and the blood decidua or spots are removed manually using either a sterile needle or pipette tip.
The TE buffer is removed by pipetting followed by adding a fresh TE buffer. Keep in mind that the CF tissue must not be discarded, accidentally. Repeat this step several times until clear pellets appear.
Using black paper or a piece of black cloth as a background can increase the efficiency of washing. In the final step, good quality, fresh, white and clear chorionic villi tissues are collected in a tube and centrifuged. The pellets are used for DNA extraction.
One can also perform this step under the compound microscope too, for better visualization.
Maternal cell contamination test:
American College of Medical Genetics, The UK Clinical molecular genetics society, the Canadian College of Medical Geneticists and Genomics and the association of Molecular Pathology advised MCC detection as a standard procedure for prenatal testing.
MCC testing includes the combination of VNTR and STR markers to examine results. Several markers that I have used are APOA, APOB, D1S80, D16S10 and a Y chromosome marker (if required). 4 to 6 makers are sufficient enough to validate the results.
(This technical information, you can’t get anywhere).
Along with the fetal sample, a maternal blood sample and a paternal blood sample (as control) are collected and processed for DNA extraction which is a process to isolate DNA from the cell. Note that a specialized DNA extraction protocol is required to extract DNA from either AF or CV.
Alleles of maternal, prenatal and paternal DNA are amplified in the standard PCR protocol by various markers and are run on either agarose gel or PAGE. PAGE gives an excellent resolution for STR. As every individual on earth is different, the results show different banding patterns for each maker.
Capillary gel electrophoresis replaces conventional gel assay which is a rapid and robust technique with many-fold higher accuracy and resolution.
Pro-tip
I have a tip for you: process the sample for detection of monogenic disorders and if similar maternal and fetal alleles are observed, perform the MCC test. This will reduce the requirement of the MCC test for every prenatal testing and also reduce the cost.
Results:
The results of MCC testing decide whether the sample can be processed further for genetic testing or not, However, if MCC is detected, the available sample can be re-washed and re-used.
Maternal cell contamination positive results:
In simple language, what should be the positive results of MCC? The results show substantial contamination in the fetal sample, right. Meaning, the prenatal testing results will not be acceptable.
When both maternal alleles match both fetal alleles for all of the markers or the majority of markers, the results are considered positive. And indicating considerable contamination. The sample can’t be processed further.
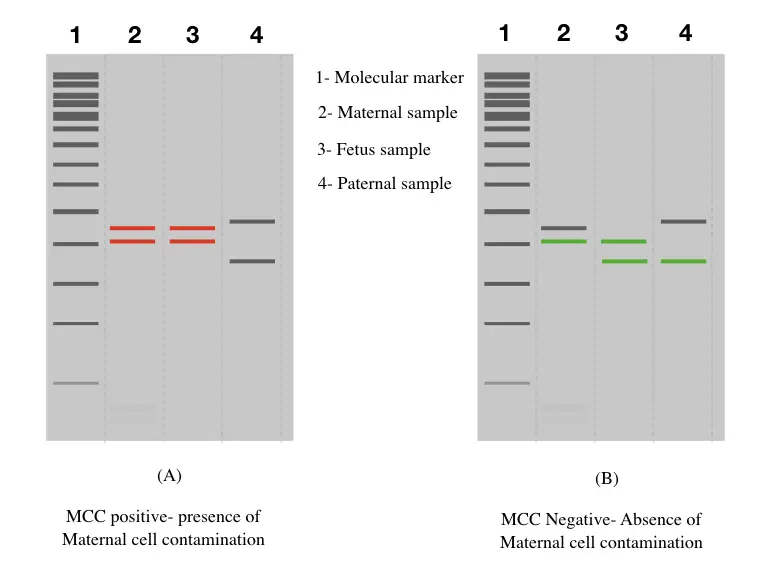
Maternal cell contamination negative results:
When only a single maternal allele, for each marker matches the fetus allele, the sample is considered as MCC negative and can be processed further for testing. Although in either case paternal alleles are used as a control.
Note:
D21S11 is one of the most important and informative STR markers with a 90% distinguishable success rate.
Wrapping up:
The MCC test is crucial for prenatal testing which is a clear indicator for sample acceptance or rejection. Nonetheless, getting MCC is highly uncertain, but largely depends on the expertise and experience of the collection personnel.
I have processed many samples without contamination and many with so much visible blood decidua. Conventional techniques in which the use of 4 to 6 markers guaranteed the results, but capillary electrophoresis is the robust and accurate option for testing.
It is advisable to test MCC first before preceding the samples for any genetic test. I hope this article will surely help you in our research. Common below and let me know about your thoughts.
Sources:
Schrijver, I., Cherny, S. C., & Zehnder, J. L. (2007). Testing for maternal cell contamination in prenatal samples: a comprehensive survey of current diagnostic practices in 35 molecular diagnostic laboratories. The Journal of molecular diagnostics : JMD, 9(3), 394–400. https://doi.org/10.2353/jmoldx.2007.070017.
Nuss, S., Brebaum, D., & Grond-Ginsbach, C. (1994). Maternal cell contamination in amniotic fluid samples as a consequence of the sampling technique. Human genetics, 93(2), 121–124. https://doi.org/10.1007/BF00210594.