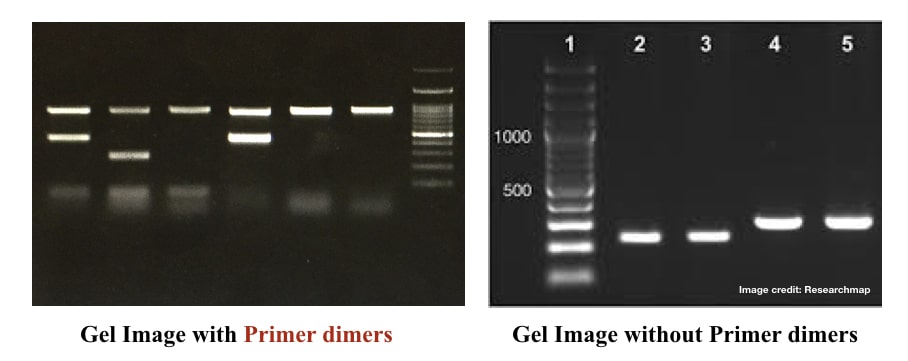
“Primer dimers are unintended short amplification products formed in the PCR by unhealthy PCR practices. This article covers common causes for primer dimer formation and troubleshooting.”
In the previous article of this series, we understood the basic concept of ‘primer dimers’ and how they form. Primer dimers have serious consequences in PCR experiments including qPCR as well. When it comes to the diagnostic approach, it becomes even more crucial to overcome it.
Dimers in PCR can usually mislead results and may cause false-positive, false-negative or unintended results. In the worst-case scenario, the results can’t be interpreted. However, optimizing a PCR reaction to avoid primer dimers synthesis isn’t rocket science.
We have to take care of some basic things, and some advanced (expert-level tips) and that’s it! The problem is solved. Let’s see what troubleshooting options we have along with some common reasons for primer dimer.
Read more:
- PCR Troubleshooting 101: How to Address Non-specific Amplification.
- PCR Troubleshooting 102: How to Address Allelic Dropout.
Key Topics:
What causes Primer dimers in PCR?
A few common causes for primer dimer in PCR are inadequate primer design, high primer concentration, low annealing temperature, Poor quality primers, prolonged PCR cycles, complementation between primers, and complex templates.
Now let us discuss each factor one after another starting with primer design.
Inadequate primer design:
Primer designing is a crucial prerequisite step in PCR. It shows us the length, efficiency, annealing temperature and product size for the selected primers. Poor primer design increases the chances of primer dimers in the reaction.
Common inadequate primer design conditions are uneven melting temperature between two primers, primers with highly complementary regions, and primers with self-complementary and overlapping regions.
Complementarity between primers:
The most common reason for primer dimer is the complementation between two primers. Complementation between even a few bases of primers results in substantial primer dimers in the reaction.
Moreover, complementation between GC-rich nucleotides increases the chances of dimer many folds.
Low annealing temperature:
The annealing temperature is a crucial factor for getting success in PCR. A low annealing temperature not only allows non-specific amplification but also primer dimer formation and extension. When primer annealing temperature is compromised, primers are involved in dimer formation.
The concentration of primers:
Higher primer concentration causes primer dimer. In the high primer-utilized reaction, the unused primers find complementation in other unused primers and form the dimer. As the primer concentration increases, more unused primers are left in the reaction, forming dimers and resulting in inadequate PCR results.
Use of PCR enhances:
We use DMSO, MgCl2, KCl or other additives that can boost our PCR reaction, right? Unfortunately, excessive or unwanted use of any PCR additive boosts the capacity of primer dimerization. Actually, adding an additive compromise the reaction conditions to facilitate amplification.
If you want to learn about where and when PCR additives are used. Read this article: PCR reaction Buffer.
Poor quality primers:
Poor quality primers also boost the dimerization activities between primers. It’s crucial to purchase high-grade and highly pure primers.
Prolonged PCR cycles:
Excess PCR cycles can unnecessarily induce primer dimer activity. Once all the ingredients are almost used the excess PCR cycles promote self and cross-annealing between primers and produce dimers.
Complex templates:
Hard templates, for example– high GC-rich template amplification are difficult to amplify. In such cases, primers, instead of annealing with the template, self or cross-anneal and form dimers. Note that high GC-rich templates are hard to amplify using normal PCR conditions.
PCR reaction preparation:
PCR reaction should be prepared using standard laboratory conditions. When the reaction tubes unnecessarily remain at room temperature, primers start annealing randomly and using the Taq DNA polymerase, synthesize short and unnecessary dimer products.
Related article: How to Prepare a PCR Reaction?
Taq DNA polymerase addition:
This point is so technical. The addition of Taq DNA polymerase in the PCR reaction also has a significant effect on primer dimer formation. Early addition of Taq DNA polymerase can synthesize primer dimers. Note that Taq DNA polymerase does have activity at room temperature as well.
Primer-Dimer Troubleshooting:
Now in this section, we will discuss some of the proven techniques to overcome or substantially reduce primer dimers in the PCR.
Accurate Primer designing:
Design primers using trusted software. I recommend using Primer 3. Check the annealing temperature, self-complementation ratio, and primer complementation before choosing the primer set.
The annealing temperature of both primers should not exceed more than 3ºC. Each primer should not have more than 3 complementary nucleotides on their 3’ ends. Read this article to learn more: PCR Primer Design Guidelines.
Avoid complementation between primer:
Manually analyze your primer set for complementation. You can also check it using the software as well. Avoid primer overlap or hairpin.
Adjust the annealing temperature:
Use the correct annealing temperature for your reaction. Avoid unnecessarily compromising it. I strongly recommend using 3 to 4 different temperature gradients before selecting the best annealing temperature for your reaction. If you see any primer dimers in a gel, set a temperature gradient.
Note that the ideal annealing temperature range is 53ºC to 68ºC. Avoid using too low or too high annealing temperatures. If you want to know how gradient reaction is prepared, read this article: Optimize Your PCR Reaction Using Gradient PCR.
Correct primer concentration:
The ideal primer concentration for any PCR reaction is 10 pM. Oftentimes, primer dimers may appear at this concentration as well, typically during low template DNA amplification.
You can use this concentration but for crystal-clear results use a primer concentration lower than this. However, I strongly advise optimizing primer concentration before using it for the actual reaction.
Some more tips:
- Use HPLC-purified high-quality primers.
- Add Taq DNA polymerase at the very end of the reaction.
- Prepare a PCR reaction on ice or at 4ºC temperature.
- Immediately transfer the reaction tubes to the ‘pre-set’ PCR protocol. Don’t even waste time switching on the machine or setting a PCR protocol.
- Avoid using excess Taq DNA polymerase in the PCR.
- Use 30 to 35 cycles for a PCR reaction.
Wrapping up:
The primer dimer is a major issue in PCR and qPCR reactions which can’t be ruled out completely. Sometimes it also creates major problems in results as well. So it’s crucial to work on reducing the dimerization process during PCR.
If you are in the diagnosis and have to repeat the same experiments every day, you have to work on primer dimer reduction. I am sure that this troubleshooting will definitely help you.