DMSO is an important PCR enhancer and an ingredient of PCR buffer. It increases the yield and specificity of the reaction. Let’s find out what its function is and, why and how to use it in PCR.
PCR is a technique to amplify DNA and give us copies of a particular fragment. A typical PCR reaction comprises ingredients like dNTPs, primers, PCR buffer, Taq DNA polymerase, Template DNA and nuclease-free water.
All components are crucial and equally important but the PCR buffer stands out as a ‘kind of reaction booster.’ It has chemicals that help reactions to occur correctly and that’s why it is required.
Common PCR reaction buffer components are MgCl2, KCl, DMSO, Albumin, (NH4)2 SO4 and various salts. Each ingredient has its own role in the reaction. But it remains ineffective for some special reactions, for example high GC-rich template.
Now talking about some atypical or special reactions like templates with high GC content, a conventional amplification setup isn’t sufficient enough here. It needs additives that can effectively amplify such templates.
DMSO is one among many other PCR additives that can help the template with high GC content. Now you have questions, what is the role of DMSO here, why and how is it used in the PCR?
In this article, we will explore optimizing hard and atypical PCR reactions using DMSO. I will explain the mechanism of DMSO working, comprehensively. I will also share my practical experience regarding what will happen at low and higher DMSO concentrations.
This article will improve your knowledge of PCR and optimizing reactions. Trust me our blog has helped thousands in their PCR venture and will benefit you too.
Stay tuned.
Do you know MgCl2 is a proven PCR enhancer? Read about it here: What is the function of MgCl2 in PCR?
Key Topics:
Structure of DMSO:
DMSO stands for Dimethyl Sulfoxide. It is an organic molecule. Take a look at the structure.
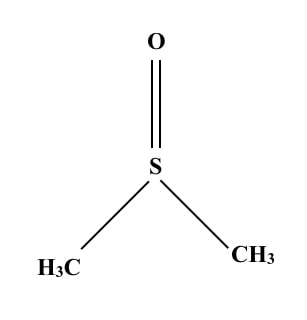
It has trigonal pyramidal symmetry. Chemically it is a 2-carbon sulfoxide having two methyl groups. Some important properties are explained here.
- It is a polar solvent and can dissolve in both polar and nonpolar solutions.
- It has higher melting and boiling points.
- The dielectric constant is ~48.9 with a pKa value of 35.1.
| Name | DMSO |
| Full name | Dimethyl sulfoxide |
| Other names | Methyl sulfoxide and sulfonylbismethane. |
| Molecular formula | (CH3)2SO |
| Molecular weight | 78.1 |
| Melting point | 19°C |
| Boiling point | 189°C |
Due to its unique properties, DMSO is often used as a cryoprotectant in medical science and also used in molecular genetics.
The Problem:
In a PCR, amplifying normal reactions with balanced AT and GC content, and normal lengthened templates is quite easy, however, longer and high GC templates can’t be effectively amplified. Such reactions are categorized as ‘atypical PCR reactions.’
As aforesaid, atypical reactions need expertise, experience, a special chemical combo and a separate setup to achieve PCR amplification. High GC DNA comes up with two common problems.
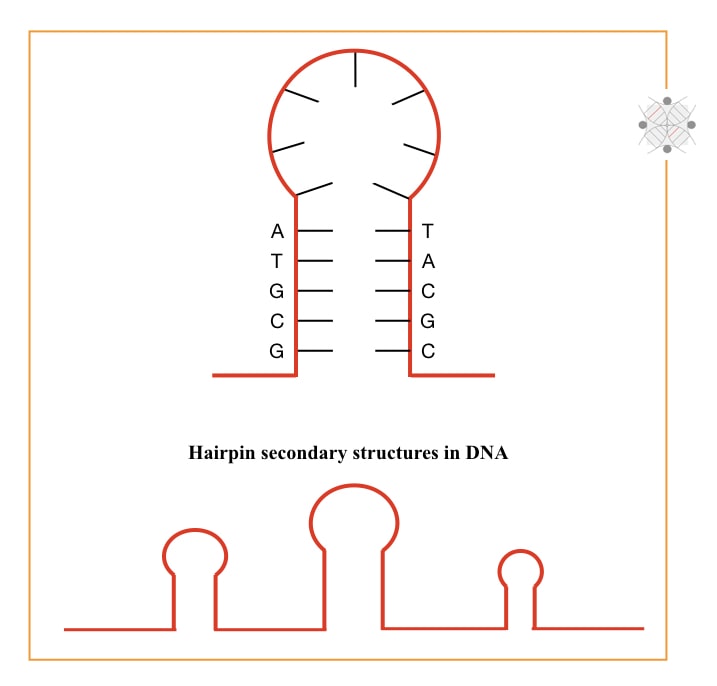
First, G and C contains triple hydrogen bonds. More hydrogen bonds stabilize the template and require a higher temperature to break them. Second, more GC-rich templates form many secondary structures in the DNA.
Secondary structures are hard to open and amplify. So we can’t get results and if so, there might be non-target amplification, primer-dimer or unwanted amplifications. Adding DMSO at a required concentration helps to overcome these problems. But keep in note that adding DMSO doesn’t warranty to prevent primer-dimers.
Role of DMSO in PCR:
Now first understand what we have to do, meaning, why we need to add DMSO. Here the reaction requires a high melting temperature which sometimes prevents accurate primer bindings and often damages the template too.
When we add DMSO, it decreases the melting temperature during the reaction. This event facilitates primer annealing. Here the DMSO binds with the cytosine base of the template and makes some conformational changes.
This causes cytosine to be more heat-labile and eventually drops the overall melting temperature for primers. In addition, DMSO reduces the strength of overall hydrogen bonding in DNA major and minor grooves and alters the structure.
One of our problems is solved.
Moving to the second one. Higher GC produces a hairpin-like secondary structure and stabilizes the single-stranded DNA. Such hairpins are hard to open and fail our reaction by not providing a complementary site for primers.
DMSO binds with the DNA and prevents reannealing of denatured DNA thereby only primers get ‘wide space’ to accurately bind at their complementary locations.
The more the primers bind at their correct complementary locations, the more amplicons we will get. So the second problem is now solved.
Conclusively, DMSO by decreasing the melting temperature and preventing reannealing, increases the specificity and yield of the reaction, respectively. That’s the exact function of DMSO in PCR.
Probably you get the point, why to use DMSO, but how to use it, what concentration of DMSO is sufficient for the job! You may wonder. Let’s see.
The concentration of DMSO in PCR:
Ideally, 3 to 10% DMSO can be used in an atypical PCR reaction. However, keep in note that for a conventional reaction which usually has 45 to 52% GC, a normal PCR reaction buffer is sufficient.
Though the composition of the buffer varies from manufacturer to manufacturer, we can’t exactly say whether or not the buffer we have contains DMSO or not. A small amount might be there.
Coming to the point, if your GC content exceeds, use 5% of DMSO in the reaction. Nonetheless, the exact concentration to match your requirement needs optimizations. Use a gradient of 4, 5 and 6% DMSO in a reaction and choose the concentration best for you.
The exact concentration of DMSO in a reaction depends on the GC content, reaction type and quality of DMSO. Use molecular grade DMSO. 5% DMSO decreases the annealing temperature up to 2.5°C.
Too much DMSO:
Why do I recommend optimizing the reaction? Because too much DMSO can adversely affect the reaction. When a concentration exceeds the desired one, it induces non-specific bindings.
Too much DMSO unnecessarily stabilizes the single-stranded DNA, reduces the melting temperature and thereby facilitates more primers binding at random locations at the target. We will not get what we want.
It actually compromise the annealing temperature for the reaction.
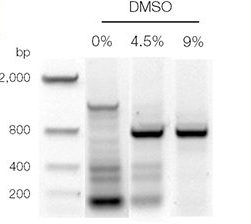
Technically, you will see many bands other than or without our target band in a gel. Keep in mind that, for sensitive experiments, the adequate amount of DMSO is so crucial as elevated concentration adds wrong bases and induces mutagenesis.
So use it wisely.
Too little DMSO:
Contrary, if we can’t use an adequate amount of DMSO, we will not get results. You lack clarity in the results. Using too little DMSO, you might have fewer, low-resolution DNA bands or nothing.
Recent findings:
Recent studies demonstrate that DMSO is a proven DNA topological agent, it changes the topology of DNA, meaning the structure. It reduces DNA supercoiling, especially, negative supercoiling of DNA.
Here it induces the catalytic action of the topoisomerase enzyme, which is a class of enzymes to relax DNA. Topoisomerase stabilizes the DNA by relaxing and reducing the coiling. DNA topology is a complex phenomenon. I have written an article on it, you can read it here: DNA topology.
Scientists use DMSO for cell proliferation, inhibition and differentiation studies. And also used as a cryopreservation agent.
Hardjasa et al. (2010) experimented on the role of DMSO and its power to induce mutagenesis. Their findings suggest that a very high concentration of DMSO affects the fidelity of polymerase and adds wrong bases and produces mutations.
Using restriction enzymes they have investigated the alterations.
Related article:
- What are the Different Components used in the PCR Reaction Buffer?
- 10 secrets to Prepare a PCR reaction.
My suggestions:
I have a huge experience in PCR and relevant technologies because I worked for years in molecular laboratories. I have my own working style and recipe to do things. Here are my suggestions on how to handle DMSO.
From my knowledge, sometimes a 52% GC-content reaction even can’t give results, for this scenario use a 3% DMSO, and if required, do optimizations.
Reactions with GC content >60% are categorized into high sensitive reactions. Use 5.5 to 7% of DMSO for this.
I often used DMSO to combine various reactions. See, the proven function of DMSO is to reduce the annealing temperature. For example, to combine two reactions at 63°C and 60°C, add 5% DMSO in the first reaction to fuse it with the second one.
Such optimization helps to perform many reactions in a single PCR, and saves time, power and resources. However, for doing these things one has to have comprehensive knowledge about ingredients, reactions and skills for performing PCR. High-end expertise is required to do this. Take expert advice.
Document your work so that it will help you in future optimizations.
You can also use Betaine as an alternative to DMSO.
Do not use DMSO if you are generating amplicons for sequencing. Because, as I explained, it will induce mutations and give false sequence signals during sequencing.
Wrapping up:
Optimizing PCR is fun but at the same time requires knowledge and discipline. DMSO is a crucial chemical in any PCR lab and today or tomorrow one has to use it. However, it is evident that when we compromise the annealing temperature, it will increase the chances of non-specific bindings too.
Conclusively, DMSO performs two important functions, preventing hairpin or secondary structure formation and compromising annealing temperature. It increases the specificity and yield of the reaction.
FAQs: