“Loss of Heterozygosity (LOH) is defined as the absence of one normal parental allele or gene (or part of the chromosome) in an organism which usually results in cancer.“
Like I say every time, our genome is mysterious, comprises enigmatic phenomenons and results in surprising outcomes; Perhaps because it’s too huge or yet not understood well. The correct understanding of one process causes a “point blank” for the following one.
For instance, when our knowledge of mutation and how polymorphism occurs in nature just getting better and better, the concept of epigenetics came to light which has once again raised a question about our knowledge and findings.
Ring chromosomes, uniparental inheritance, Loss of heterozygosity, etc are some of those questions; unanswered and complicated. We will start with the most complex and difficult mechanism which is the loss of heterozygosity in this article. But let us start with some basics so that we can strengthen the foundation for the present article.
We get our genome from our parents, meaning our whole set of DNAs. As the genome is diploid (in a copy); the father contributes one set and the mother contributes another set. All 23 pairs of chromosomes are inherited in this fashion and thereby genes too.
For example, if we take an example of the Hb gene, among two Hb/Hb copies, each parent inherited each copy. Sometimes mutations change the genetic constitution of any of the gene copies and create three different conditions for an organism, viz,
- Hb+/Hb+ – Homozygous Normal alleles
- Hb+/Hb- – Heterozygous alleles
- Hb-/Hb- – Homozygous mutant alleles
But let me tell you, what if one copy from a single parent can’t be inherited? especially, emphasizing the heterozygous condition, what happens if one allele from any of the parents will not inherit?
The organism will not acquire heterozygosity, meaning, a loss of heterozygosity. In the present article, I will explain to you the concept of loss of heterozygosity, its meaning and its association with cancer.
Stay tuned.
Key Topics:
What is Loss of Heterozygosity (LOH)?
Definition:
When events like deletion, duplication or gene conversion prevent the inheritance of a gene, gene(s) or a chromosomal part from one parent, the phenomenon is defined as loss of heterozygosity.
Loss of Heterozygosity is a kind of genetic polymorphism (not a true mutation in my opinion), causing cancer. It is abbreviated as LOH and can be scored using computational and mathematical calculations.
Loss of heterozygosity meaning:
Let us understand conceptually by taking an example of the P53 gene. The P53 gene having a location on chromosome 17 is a proven tumor suppressor gene and well-studied with loss of heterozygosity.
It has a definite role in various types of carcinoma and hence is an important cancer marker. When parents inherit one normal and mutant copy of the P53 gene, though the individual has a genetic predisposition for cancer, it will usually remain unaffected.
However, the mutant P53 allele can’t make the correct protein and thereby loses its function, but the normal allele works finely.
(we are talking about the heterozygous condition- P53+/P53-).
Unfortunately, during the 17p deletion, the normal allele for the P53 gene is lost and resulting in the loss of heterozygosity. In this condition, the mutant P53 allele can’t manufacture the protein, consequently, losing its control over the process of cell division and leading to cancer.
Do you know?
“~62% of patients diagnosed with P53 mutation get cancer has LOH by chromosome 17p deletion.”
– Faults et al., 1992.
On a broad level, it changes the number of copies (copy number variation). Read more on CNVs: What is Copy Number Variation and How to Detect it?
The LOH is also reported for normal homozygous conditions as well. In this scenario, for homozygous normal, if an individual lacks one normal allele, its likelihood of getting cancer increases as it has only one normal allele.
Whenever the single normal allele gets mutated during an individual’s life, he or she will acquire cancer because it doesn’t have another allele to refill the normal function. Furthermore, at the epigenetic level, sometimes insufficient gene expression of a single normal allele also causes sudden carcinoma.
Take a look at the picture here,
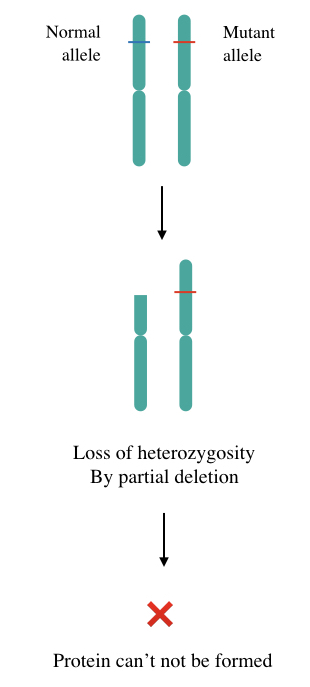
So the loss of heterozygosity not only causes cancer but also increases the predisposition of genetic cancer by genetic anticipation. Two common mechanisms by which the LOHs occur are Copy-loss LOH and Copy-neutral LOH.
Copy-Loss LOH:
The present mechanism of LOH is simple, well-understood and straightforward. Here, in a cancer cell, by deletion of chromosomal arm or allele, the LOH occurs (which eventually causes cancer).
On a genome-wide spectrum, it causes change at the CNV level, meaning the overall copy number will change.
Copy-neutral LOH:
Copy-neutral LOH is the complex mechanism of LOH. Here, the number of copies of the allele remains the same but still, it causes cancer by LOH. Studies at a molecular level revealed that duplication of the remaining allele fills the gap that occurred by the deletion of another allele. So technically it’s a “copy-neutral” but still, one parental allele isn’t present in this type of LOH.
Zang and Tobias, 2021 in their paper entitled, “Targeting loss of heterozygosity: A novel paradigm for Cancer”, further explained that the CN-LOH occurs by uniparental disomy. However, the reason for the CN-LOH occurs by complete or partial deletion.
Loss of heterozygosity and cancer:
Loss of heterozygosity has a significant role in developing various types of cancers. It is majorly reported in cancers caused by the tumor suppressor genes like P53, BRCA, HLA, DPC4, WT and MEN1. Here is the list and percentage of HOL.
| Cancer type | % of LOH | Tumor suppressor gene |
| Breast cancer | 42.1% | HLA |
| Brain tumor | 62% | P53 |
| Liver cancer | 24.1% | HLA |
| Cervical cancer | 59.7% | HLA |
| Colorectal cancer | 43.8% | HLA |
| Sporadic colon cancer | 51% | DPC4 |
| Endometrial cancer | 23.8% | HLA |
| Gastric and esophageal cancers | 39.6% | HLA |
| Glioblastoma | 20% | TRIM3 |
| Kidney cancer | 32.1% | HLA |
| Non-small-cell lung cancers | 53.7% | HLA |
| Ovarian cancer | 38.8% | HLA |
| Pancreatic cancer | 32.8% | HLA |
| Wims’ Tumor | 5% (for 1p and 16q) | WT |
| Anaplastic oligodendrogliomas | 50% (for 1p and 19q) | |
| Parathyroid tumors | 100% (11q13) | MEN1 |
There are two types of genes when mutated, causing cancer, are tumor suppressor genes and oncogenes. Tumor suppressor genes control the progression of cell division while the normal form of oncogenes (proto-oncogenes) regulates the process of replication.
Mutation in either type of gene results in uncontrolled cell division which is cancer.
Tumor suppressor genes are important markers for LOH study and suppress tumorigenesis by normal functions. Events like CNVs or genetic rearrangements abrupt the normal function of TSG by LOH.
Notedly, the LOH is one among many types of mutations reported in various types of cancer. LOH is also evident in both hereditary and non-hereditary types of cancers. In hereditary cancers, germ cells acquire LOH early during fetal development whereas, in non-hereditary cancers, somatic cells acquire LOH by genetic anticipation.
Studies found that LOH causes cancer by various means, common possible ways are,
- The Inactivation of a single normal gene
- Inheritance of only a single mutant gene
- Mutation acquired by a single gene
- Underexpression of a single normal gene
Noteworthy, in any of the above-listed conditions, a correct governing protein can’t be formed. Take a look at the two classic and well-studied examples of LOH.
Fact:
“Women having a germline LOH have an 85% risk of breast and/or ovarian cancer throughout their life.”
–Neff et al., 2017.
LOH in retinoblastoma:
Retinoblastoma is a type of malignant tumor caused by the loss of heterozygosity in the Rb1 gene. Rb1 gene which has been located on chromosome 13 at 13q14, encodes a protein p110RB. It has a significant role in the regulation of the cell cycle and is a tumor suppressor gene.
Loss of copy number at 13q14 by deletion or copy-neutral LOH results in retinoblastoma. Among two mutational M1 and M2 events, M2 occurs predominantly by LOH (Zhu et al., 1992). Stahl et al., 1994 further suggest that LOH is reported in both hereditary and sporadic retinoblastomas.
LOH in breast cancer:
Breast cancer is a tumor of the breast that occurs from some part of the breast and gradually spreads to the whole breast and to another breast. Wang et al., (2004) reported a profound role of LOH in up to 60% of breast cancer cases. Some common CNVs are 17p, 17q, 16q, 11q and 14q.
However, BRCA1 and BRCA2 are two candidate genes involved in causing breast cancer as well as ovarian cancer. Besides, loss of heterozygosity by 1p, 1q,3p, 6q, 7q, 8p, 11p, and 22q CNS are also reported in various other reviews (Miller et al., 2003).
Maxwell et al., 2017 also reported LOH in BRCA1 and BRCA2 genes in 7% of cases having a definite association with breast and ovarian cancer.
Read more: Breast Cancer Genetics- Genes, Mutations, Inheritance, Testing and Diagnosis.
Detection of LOH:
Microsatellites (STRs) and SNPs are common markers used for the detection of loss of heterozygosity using techniques like the Whole-genome microarray, chromosome microarray, whole-genome sequencing, SNP arrays and FISH.
Preliminarily in the detection, the change in the number of microsatellites is taken into consideration for locating the deletion or partial deletions. For example, D1S199, D1S186, D1S162 and D1S312 etc are some microsatellite markers present on the p arm of chromosome 1.
If any deletion comprises any of these markers, it shows the change in the number of copies that manifested that alleles present on that arm or in close proximity of any of these markers are lost.
Wrapping up:
Loss of heterozygosity studies are comparatively new to us, however, it is an important cancer marker, recent studies show. More whole-genome studies and genome-wide data are required to collect more information regarding the LOH.
Unfortunately, LOH studies aren’t yet fully available for cancer studies at the diagnostic level. In the future, it would be available and also can be used to evaluate the predisposition to cancer.
Sources:
Fults, D., Brockmeyer, D., Tullous, M. W., Pedone, C. A., & Cawthon, R. M. (1992). p53 mutation and loss of heterozygosity on chromosomes 17 and 10 during human astrocytoma progression. Cancer research, 52(3), 674–679.
Stahl, A., Levy, N., Wadzynska, T., Sussan, J. M., Jourdan-Fonta, D., & Saracco, J. B. (1994). The genetics of retinoblastoma. Annales de genetique, 37(4), 172–178.
Zhu, X., Dunn, J. M., Goddard, A. D., Squire, J. A., Becker, A., Phillips, R. A., & Gallie, B. L. (1992). Mechanisms of loss of heterozygosity in retinoblastoma. Cytogenetics and cell genetics, 59(4), 248–252. https://doi.org/10.1159/000133261.
Wang, Z. C., Lin, M., Wei, L. J., Li, C., Miron, A., Lodeiro, G., Harris, L., Ramaswamy, S., Tanenbaum, D. M., Meyerson, M., Iglehart, J. D., & Richardson, A. (2004). Loss of heterozygosity and its correlation with expression profiles in subclasses of invasive breast cancers. Cancer research, 64(1), 64–71. https://doi.org/10.1158/0008-5472.can-03-2570.
Miller, B. J., Wang, D., Krahe, R., & Wright, F. A. (2003). Pooled analysis of loss of heterozygosity in breast cancer: a genome scan provides comparative evidence for multiple tumor suppressors and identifies novel candidate regions. American journal of human genetics, 73(4), 748–767. https://doi.org/10.1086/378522.
Maxwell, K.N., Wubbenhorst, B., Wenz, B.M. et al. BRCA locus-specific loss of heterozygosity in germline BRCA1 and BRCA2 carriers. Nat Commun 8, 319 (2017). https://doi.org/10.1038/s41467-017-00388-9.
Jian’An Huang, Jie Hu, Xiaorui Fu, Xiuqing Zhang, Fan Tong, Xinhua Du, Ziwen Luo, Yuting Yi, Yan-Fang Guan, and Xuefeng Xia. The prevalence of HLA LOH across 10 cancer types in Chinese patients. Journal of Clinical Oncology 2020 38:15_suppl, 3124-3124.