“The PCR test is performed to get millions of copies of template DNA for performing different genetic applications using the heat dependent rapid amplification method.”
Why we always give importance to the PCR? Because it is a key step in genomic as well as research techniques starting from RFLP to the NGS. This is the reason why doing a PCR test is very important.
PCR is one of the common technique employed in the molecular genetic labs. By using the amplification method, mutations present in a template DNA can be identified.
The PCR test is applicable mainly in,
- Diagnosis of different single-gene disorders
- Identification of mutations
- Genotyping
- DNA fingerprinting and microbial identification
- Environmental DNA analysis
- Metagenomic analysis
Besides this, it is also used for performing so many different functions. You can read 50 different application of PCR test in our previous article: 50 Powerful Applications Of PCR.
The PCR technique was developed by the Karry Mullis in the year 1983. He had amplified DNA with the help of the thermostable Taq DNA polymerase.
Denaturation, annealing and extension are three major important steps in PCR in which the DNA is denatured, primer amplifies and the Taq DNA polymerase extends DNA during each step, respectively.
For more detail on the entire process, protocol and other related information, please our article on PCR: Polymerase Chain Reaction (PCR)- Definition, Principle, Steps, Procedure, Protocol, Applications and Types.
For performing the PCR test- pre-planning, proper reagent preparation, the quantity of each ingredient and sterile environment must require for getting excellent results.
In this article, we will give you 10 important tips which are essential for performing a PCR test. By using these tips you can achieve a good amplification, 100% guaranteed. Here we are giving you tips which are very important to do PCR effectively.
Key Topics:
10 tips on how to do a PCR test:
1: Preparation of working solutions:
All the reagents used into the PCR come in 10X concentration. We have to prepare a working solution for primer, mastsermix, template DNA and PCR buffer. The guide for the preparation of 1X from the 10X solution is given into the individual articles. Read our previous articles of PCR.
2: Sterile the PCR workbench:
Sterility is one of the important factors in performing any PCR test, make the workplace or laminar airflow sterile before preparing the PCR reaction. A small trace of foreign DNA can result in false-positive or false-negative results.
Contaminants and fragments of other DNA are present in the molecular lab which is obvious. And it is normal of course! because we are doing experiments on DNA. Even, the DNA may present in the atmosphere in the form of aerosols.
Make the cabinet contaminant-free by UV sterilization and by the alcohol. Clean all the pipettes and the bench of the laminar with the alcohol.
3: Thaw the reagents:
Every PCR reagents are stored at -20°C. Mastermix, Taq DNA polymerase, primers and template DNA are stored at -20°C, at that temperature, it remains stable and inactive for a longer period of time. while preparing the reaction, take all the reagents into the rack and thaw it by your hands before starting the reaction preparation.
Thaw it until it becomes liquid and place it at 4°C for some period. If proper thawing is not performed the concentration of components present into the reagents might not distribute properly therefore always thaw reagent properly before performing a PCR test.
4: Labelling the PCR tubes:
Some scientist takes it as granted but it is the most important point “label each PCR tube” properly. Do it with the water-resistant marker pen and place it in ascending order.
Label tubes like 1,2,3…. And positive control as PC, negative control as NC and internal control as IC. labelling facilitates ease during the reaction.
5: Controls in the reaction:
Control tubes are very important for reference to the result. We have to compare our results with the other results, called as a control. So we have to prepare three different control tubes.
Internal control: internal control is a type of control which amplifies the DNA other than our target sequence which is present in all the PCR tubes along with our targeted sequence. That means we will get two bands at the end of the PCR reaction. One with our target sequence and another with the internal control.
Select internal control which gives amplification between 500bp to 600bp so that we can distinguish it from our result. Select the less complicated sequence as internal control hence with less GC content, no hairpin and with lower annealing temperature.
Positive control: the positive control contains the DNA band of our target sequence without internal control. This will specify whether the internal control will hinder in the amplification or not. It has an only single band.
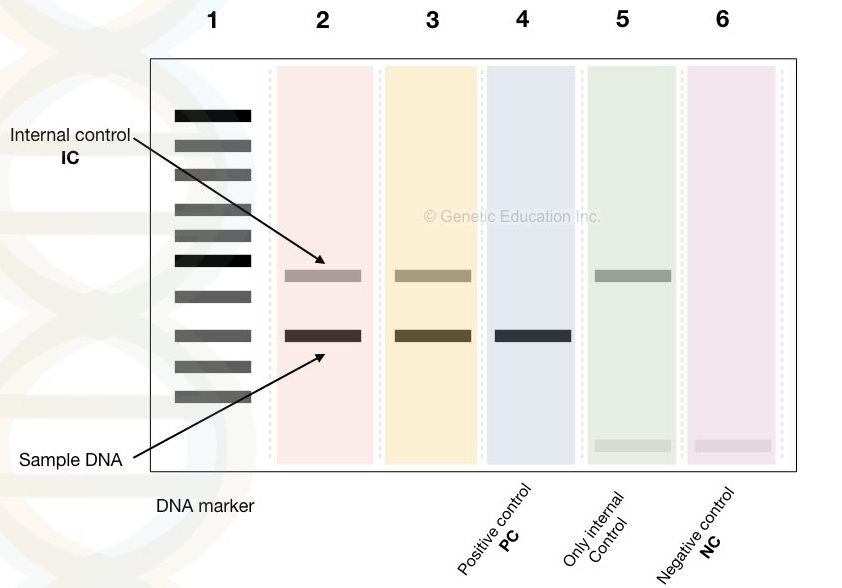
Negative control: negative control includes all the ingredients of PCR but not the template DNA. This means that we can not get any types of amplification in this tube, though it has all the ingredients.
Suppose if we get any amplification, it indicates that any of the components used into the PCR reaction might be contaminated with other DNA. Because of these reasons control tubes are always required in each PCR reaction.
6: Wear gloves and lab coat:
Chance of contamination is common in each and every step during the PCR test. Always wear gloves, a mouth cap and lab coat. This will also protect you from the infection and protect you from the hazardous chemicals as well.
Wear gloves from the beginning, starting from thawing.
7: Follow your own pattern for a reaction:
If you are planning for doing genetics as your carrier you have to develop your own pattern of working. Though we have an SOP for working in a lab, still developing our own method of working will boost our working efficiency.
For example, for doing PCR, I have always followed the pattern of mastermix> forward primer> reverse primer> water> DNA. This is my own SOP, I always follow this pattern. You have to decide that in which pattern you are conformable.
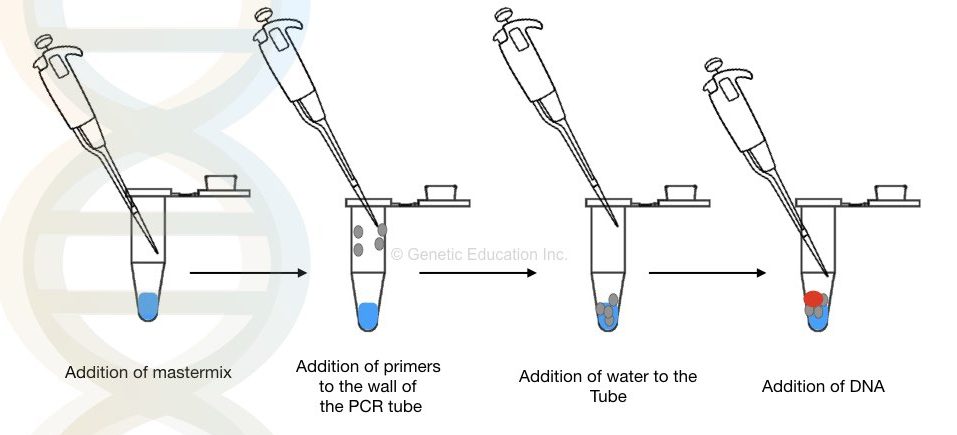
First, I drop a mastermix in the bottom of the tube then I add the forward and reverse primer on the side of the PCR tube. So that I can see the drops of the primer on the side of the PCR tube. Add primers for internal control in the same manner.
Followed by the primer addition, I add water into the tube which takes primer drops with it into the bottom of the tube.
Finally, at last, I am adding the template DNA. Adding DNA, at last, minimise the chance of starting the reaction early before the PCR. This pattern of reaction always gives me excellent results without any failure.
8: pipetting:
A very important thing in the PCR is pipetting. In PCR, we are adding 1 to 2µL of reagents hence a µL of mistake fails our PCR reaction. So it is important to do pipetting precisely
There are two pressing positions for the pipette. Try to inject chemicals in a single press avoid the second pressing, especially in case of PCR. If some of the reagents remain inside the tip of the pipette after the first press, your pipette needs calibration.
Forward pipetting is recommended, avoid back pipetting. Use a new tip all the time, never reuse the tip for another addition. It will contaminate the reaction.
Read more on PCR:
Bonus tips: none of the instruments is 100% accurate. So if we are adding a µL of primer, some amount of primer remains into the tube and this will become a major problem in PCR. To avoid this add 1.1 or 1.2 µL of primer. This will add exactly 1µL to the reaction (not scientifically recommended, it is my own experience, try it).
9(a): Spin it and win it:
Now our PCR reaction is ready. Next, we have to mix all the reagents evenly in the tube. For that take a PCR tube, close the tip of the tube and gently tap it with the finger. Do it for all the tube and spin it at low RPM.
High-speed centrifugation leads to clumping of the reagents to the bottom. Spin it gently but not vertex it. Vertexing will fragment the DNA present into the PCR tube. Avoid vortexing.
Keep observing the tube that neither a single drop of reagents remain on the sidewall of the tube (you can also use a pinch of glycerol or BSA).
9(b): Storing it back:
If any of the reagents remain at room temperature, its activity decreases over a period of time. So quickly wrap all the tubes of the reagent with the parafilm and place it back at -20°C.
10: Putting the tube into the machine:
Turn on the machine and set the protocol for your reaction and put the tubes as shown into the figure below, in one of the following methods.
While putting the tubes into the machine observe several things,
- None of the caps of tubes remains open
- No air bubbles are there in any PCR tube
- Roughly observe the quantity of the reaction mixture
- None of the tubes is leaking

Important points:
If your day is very busy in a lab with 3 or 4 PCR reaction a day, every time thawing and freezing reagents will affect their activity. Instead, make aliquots; 100µL mastermix, 10µL primers and even 200µL of DD/W and store it at 4°C.
We know that this will be used within two days so it is fine. The whole stock of 500µL or 1000µL will remain safe. You can use reagents for longer periods of time by avoiding repeated freeze/thawing.
Never heat any of the reagents for thawing, not even a gentle heat. Heating will surely inactivate your reagent.
Practise to do reactions on the ice, chilling will restricts the early activity of Taq DNA polymerase.
Preparation of 15 to 20 reaction is fine by practising this SOP for more reaction such as 80 or 90 tubes, use a multichannel pipette. Multichannel pipettes make the work easy for a large number of reaction.
For that, if you have an 8 channel pipette prepare each reagent in the series of 8 tubes (8 mastermix, 8 water, 8 forward and reverse primer). It will make your work easy.
Some of the interesting articles:
Conclusion:
The PCR is used for amplification as well as provides high-quality amplicons for DNA sequencing and microarray. Thus learn to perform it properly.
These are some of the routine and important tips or point from my side, that is crucial for getting excellent PCR results. Remember, perform each step sincerely, use high-quality chemicals and always be a competitor of your own. This will make you a better scientist.
Source: