“False positive and false negative results in PCR are two critical problems. In this article, learn about these two common PCR errors, how it occurs, and how to overcome them.”
PCR is an essential technique in molecular genetics having the primary function to copy the target DNA sequencing. However, modifications like the qPCR help quantify the amount of template or target DNA present in the sample.
A gel or a computational graph must be read to study, understand and interpret the PCR results. And that I, perhaps, think is an easy task for the budding geneticist. But do you know sometimes, PCR results play tricks on us?
You heard it right!
Sometimes, positive results are not positive, and negative results are not negative. Meaning, a PCR result shows the presence of a template when it’s not or fails to determine the template when it’s there! Confusing?
Such results are known as false positive and false negative results in PCR which lead to misinterpretation. In this article on our serious PCR troubleshooting, we will learn about the concept of false positive/negative results. In addition, I will also explain how it occurs and how to overcome it.
Stay tuned.
Key Topics:
False PCR Results?
False PCR results refer to misleading or incorrect outcomes obtained during any PCR experiment. Two types of false PCR results are false positive PCR results and false negative PCR results.
False results are common PCR anomalies reported in conventional PCR, RT-PCR, qPCR and other variants. Let’s discuss each, one by one.
False positive PCR results:
A false positive PCR result is a wrong result but suggests the presence of target DNA when it’s not there. Meaning, what the results show is actually not our target DNA, it’s something else.
Such results occur when PCR amplifies or quantifies the same type of wrong template or identical DNA sequence by error. Here, the wrongly amplified DNA may behave like the target DNA in size or number of amplicons (in the case of qPCR).
Let’s take a simple example.
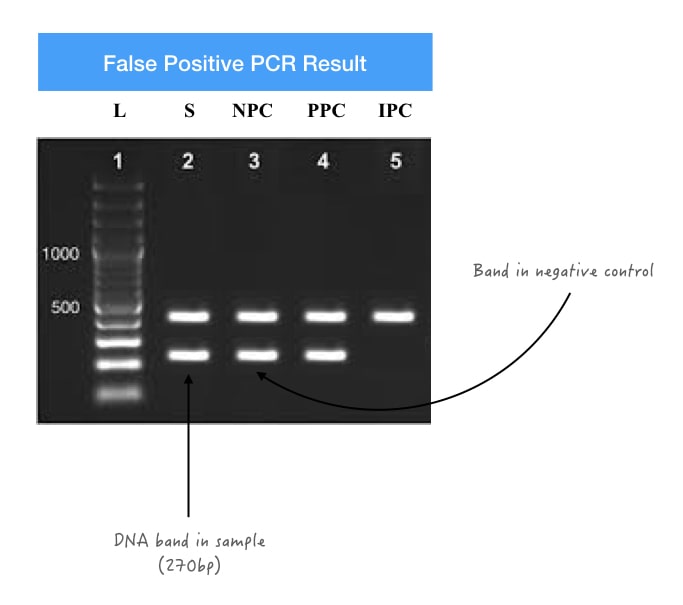
Suppose we want to amplify a 270bp long high GC-rich template. We have a band very nearer to ~270bp but not exactly 270bp or we have the exact same DNA band as the 270bp template and also have a PCR-negative control band.
These both cases are clear cases of false positive PCR results. How is it interpreted? Negative control doesn’t have any template DNA and thus, does not show any amplification. So when a slight light, dark or prominent DNA bands appear in the negative control, it shows contamination.
This demonstrates that the amplified product is contaminated with a previous product or another experiment. Or the primer set has amplified a similar type of sequence. Common causes for FPRs in PCR are explained here.
Reasons for false positive results:
Cross-contamination, unintended DNA amplification, non-specific amplification and primer dimers are common reasons for false positive PCR results.
Cross-contamination:
Cross-contamination is the most common cause of false positive results in which during the sample preparation, reaction preparation or sample addition, amplicons or DNA from the previous reaction or experiment get amplified, instead of our target DNA.
Cross-contamination occurs through the reuse of gloves, PCR tubes, pipette tips or other utilities.
Unintended DNA amplification:
It is also possible that unintended bacterial, viral or any foreign DNA present in the air or working surface as DNA aerosol may amplify and produce false positive results.
Non-specific amplification:
Non-specific amplification occurs when the set of PCR primers amplifies the DNA other than our target. The interpretation part becomes even more difficult when it amplifies nearly similar-sized fragments.
Non-specific amplification misleads the results as false positives. To learn more read this article: PCR troubleshooting 101: How to address non-specific amplification?
Primer-dimer:
Primer dimers are short and unintended PCR products. Dimers contribute to false positive results, especially during the amplification of a short DNA fragment of up to 100 bp. Dimers are short amplicons of 50 to 100 bp, depending upon the primer sequence complementation.
So, when our amplicons fall under this range, the presence of a primer dimer misleads the results as false positives.
Background noise:
Particularly, in the qPCR reaction, the background noise misleads the results. Background noise is the non-specific signal in qPCR. When the target signals are too weak or very near to the detection range, the presence of background noise can be misinterpreted as positive amplification.
However, background noise can not interfere when the template signal is so strong.
How to overcome false positive results?
Some proven suggestions are listed here to overcome this kind of result.
PCR controls:
To detect either type of PCR anomalies include controls during the PCR reaction. A negative PCR control (NPC) is a strong marker to understand and detect false positive results.
Put simply, the negative control is a reaction without template DNA which means that no amplification will occur in this reaction. So any DNA band in this reaction shows cross-contamination or unintended DNA amplification.
If you wish to learn more about controls in PCR, you can read our previous article: Types of PCR Controls.
Correct primer design:
Primer design is a crucial process in any PCR. Carefully design PCR primers so that they can only amplify our target or template DNA. In addition, the primer set should have a low dimerization capacity.
Avoid cross-contamination:
As mentioned, cross-contamination is a major cause of false-positive amplification. To avoid it, maintain strict aseptic conditions and avoid re-using any utilities. Keep in note that previous amplicons or other DNA should not interfere with our PCR reaction.
These guidelines should be followed.
- Avoid re-using utilities.
- Store previous amplicons or DNA far from the reaction preparation area.
- Follow proper sterilization.
- Differentiate work areas.
- Use filter tips.
- Use a different set of reagents.
Correct annealing temperature:
Annealing temperature also plays an important role in false positive results. When the annealing temperature is compromised or the reaction occurs at a lower annealing temperature, it produces many non-specific products.
Such non-specific products resemble the control DNA and cause false-positive results. Use the correct annealing temperature by setting a grading PCR reaction.
False Negative PCR results:
When a PCR reaction fails to amplify the target region, even if it is present in the sample, such errors are referred to as false negative results. Meaning, it shows negative results, which are actually not negative.
For instance, suppose we want to amplify the 2000 bp long high GC-rich template. As we know that a high GC-rich template is difficult to amplify, and the chances of amplification failure are very high in this condition.
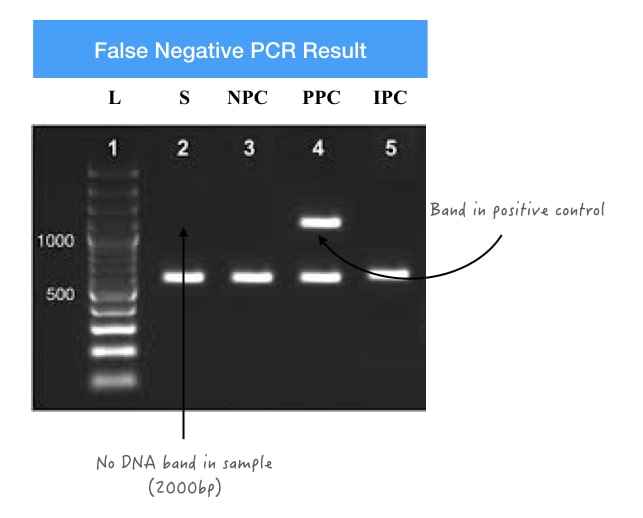
So, when any PCR condition is compromised (reaction preparation, reaction conditions, annealing temperature, etc) it fails to amplify the template even though it’s present. Such results are known as false negative results. And can be determined using a PCR-positive control.
Reasons for false negative results:
PCR inhibitors:
PCR inhibitors are the major factors that contribute to false negative results. Chemicals used during DNA extraction and other enzymes are common PCR inhibitors that block the amplification.
A few common PCR inhibitors are SDS, uria, phenol, cell wall proteins, proteases and nucleases.
Related article: Effect of PCR inhibitors on PCR amplification.
Sequence variations:
The second most common reason is sequence variation or mutation in the target sequence. Our primers are specific to the target sequence. When any alteration occurs in the sequence, it fails to anneal primers correctly, eventually fails amplification and produces false negative results.
Poor quality DNA template:
Degraded, contaminated or poor quality DNA template fails the PCR miserably. Inefficient DNA extraction, rigorous DNA handling, mechanical DNA shearing, etc are common reasons for a poor-quality DNA template.
A good quality DNA template should have a purity of ~1.80 and a quantity sufficient for amplification.
Suboptimal PCR conditions:
Suboptimal PCR conditions such as very high annealing temperature, inappropriate denaturation, inefficient Taq DNA polymerase, buffer composition and PCR cycling conditions also result in false negative results during PCR.
Pre-preparation errors:
The commonest reason for amplification failure is pre-preparation errors like– forget to add the template, Taq DNA polymerase, dNTPs or primers in the reaction. Although this can be identified easily as the control also fails to amplify in this situation.
Besides, low concentration of template, primer or dNTPs or inaccurate primer design can also contribute to false negative results in PCR.
How to overcome false negative results?
Purify the template DNA before using it in the reaction.
Use the appropriate concentration of primers, template DNA, dNTPs, Taq DNA polymerase and buffer.
Minimize the interference of PCR inhibitors.
Design appropriate PCR primers.
Optimize PCR conditions like– annealing temperature, cycling conditions or concentration of ingredients for the reaction.
Use PCR controls. Positive and internal PCR controls are a good marker to understand, identify and resolve false negative results.
Sequence template DNA to understand if any mutation is present in the DNA or not.
Related article: PCR Troubleshooting 102: How to Address The Allelic Dropout.
Wrapping up:
False positive and false negative PCR results are common amplification anomalies. To accurately report the PCR or qPCR results, one has to learn to identify and overcome them. PCR controls are an excellent and popular option to resolve the false results scenario in the PCR.
However, it’s also pivotal to understand and interpret the PCR control. To do so, you can read our article on this. I hope this article makes sense to you and will help you in your PCR experiments.