“Sodium acetate is a sodium salt having a subsidiary role in DNA Extraction. It helps alcohol for better precipitation. But how does it work? What concentration of NaAc do we need for precipitation? Let’s find out.”
I like DNA extraction the most. The reason is that it’s complicated, needs constant optimization and varies from sample to sample. For instance, the protocol for blood DNA extraction can’t work for plant DNA extraction.
And that’s what a scientist needs! It has infinite scopes to learn new things. We can prepare different combinations of lysis buffer, use different homogenization techniques, various chemicals, and physical methods, and can even prepare a uniform and simplified protocol.
I know, many easy ready-to-use kits are there but trust me those have flaws too. Any way. If you understand DNA extraction, chemical composition and the role of chemicals, you can optimize the process and sometimes replace some chemicals with others.
For instance, I have used isopropanol and ethanol both for precipitation. However, to know which works better, you have to perform optimizations and that’s “research”.
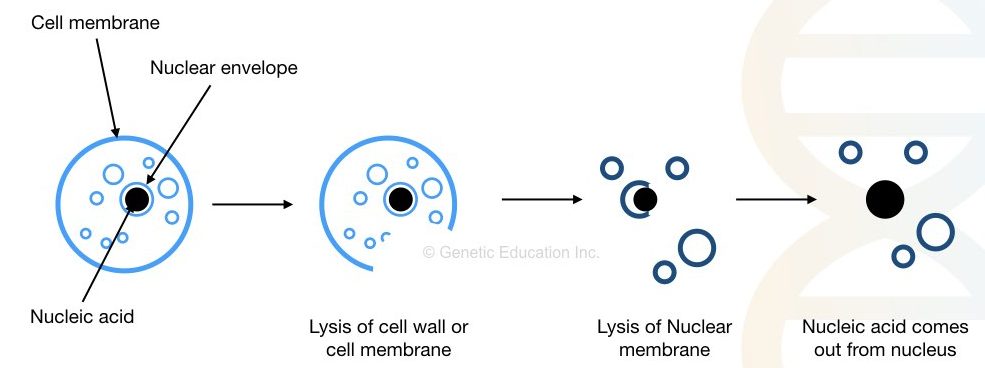
Common steps in any DNA extraction protocol are cell lysis, precipitation, washing and DNA elution. Especially, the precipitation is a process in which we can see DNA in a visible form and thus is crucial.
As we said in other articles, any alcohol can precipitate the DNA but isopropanol do the best job. However, another chemical sodium acetate is also used to increase the quality and yield of DNA precipitate.
Let’s explore how we can use sodium acetate, what concentration is required for precipitation and what is the chemistry behind its working. This article will boost your learning of DNA extraction. Trust me it will add more value to your genetics portfolio.
So stay tuned.
Key Topics:
What is Sodium Acetate?
Sodium acetate is a type of sodium salt with acetic acid. The chemical formula is NaCH3COO. Here are some of the properties of sodium acetate.
| Name | Sodium acetate |
| Chemical formula | NaCH3COO, CH3COONa |
| Molecular formula | C2H3NaO2 |
| Applications | Biotechnology, food industry, cement industry and buffer preparation. |
What is the role of Sodium Acetate (NaAc) in DNA Extraction?
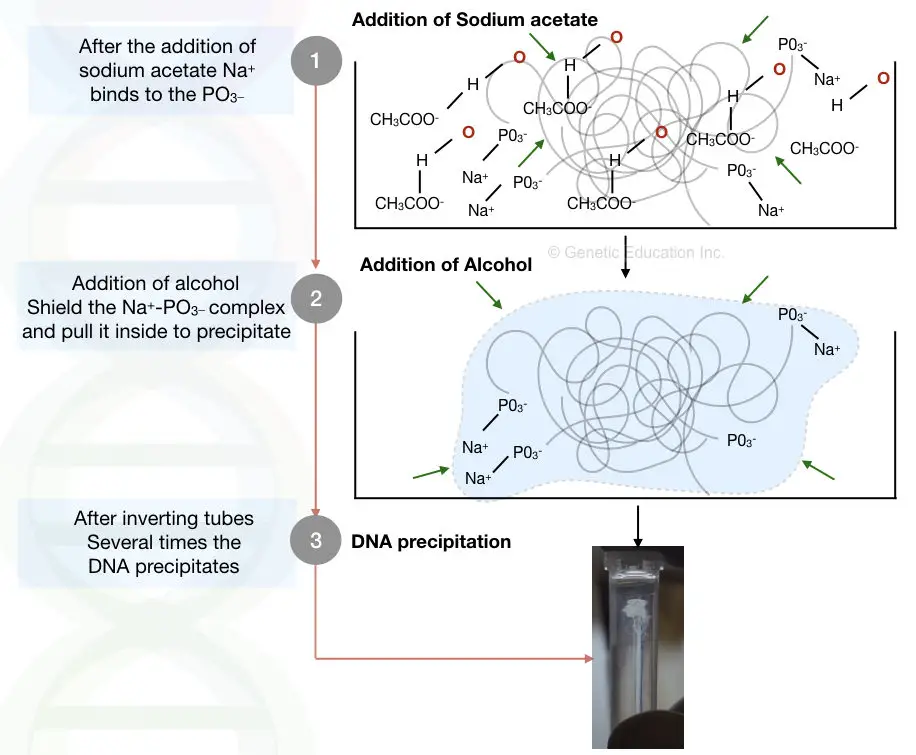
Sodium acetate salt is used in DNA extraction to precipitate the DNA. In the solution, the positive charge Na+ of the NaAc competes with the positive charge of water to bind with the negative charge of DNA.
It electrostatically interacts with the PO3– of the DNA and decreases the hydrophilicity of the DNA. Notedly, alcohol helps in this process by protecting the Na+ and PO3– complex from the water, making it less stable and precipitating DNA.
But here is a problem.
DNA is polar and so water is! Therefore it has more tendency to interact with water and become soluble. So in a race with sodium acetate, water as a polar molecule wins the race in the end. Big problem.
When we add more alcohol, along with sodium acetate, more water molecules are neutralized by alcohol (positive charge). As a result, more positive charges of sodium acetate can interact with the DNA and precipitates it. The sodium salt is important, therefore.
Yet another factor that favors the use of NaAc is the charge positivity. Adding NaAc is a wise decision because the positive charge of sodium acetate is very strong compared to the weak and partial positive charge of water.
Technically, it is vital to understand that pH doesn’t have any role here. But ideally, sodium acetate with or above pH 5.2 (acidic) gives excellent results as DNA usually needs an acidic environment.
What concentration of Sodium acetate is required for precipitation?
We use 1/10 volume of sodium acetate for the precipitation. In a layman, we can say we use only a pinch of it. 3M solution of NaAc at pH 5.5 is prepared and 0.3M final concentration (1/10 vol) is used in the precipitation.
If you have a solution of 3M, you can directly add 100 µl to the reaction per 1ml of alcohol. If you want to add 2ml alcohol then add 200 µl.
Avoid using too much sodium acetate in the reaction, it will precipitate protein and eventually decrease the purity of the DNA.
My suggestions:
I am a geneticist, If you are our regular reader, you might know that. I worked for years in molecular genetic labs. I have used and optimized many DNA extractions and PCR protocols. So I have decent work experience and hence have some personal optimization tips for students.
Here are some,
- Avoid adding sodium acetate directly in a powder form.
- RNA can also be precipitated by using sodium acetate with the same volume (and even protein can).
- Other sodium salts like sodium chloride and other salts like lithium chloride can be used as a replacement for sodium acetate.
- Increasing the volume of salt for precipitation doesn’t warranty increasing the purity and yield of DNA. So use it wisely.
List of salts used in DNA precipitation
| Salt | Chemical formula | Stock concentration | Final concentration |
| Sodium acetate | C2H3NaO2 | 3M | 0.3M |
| Ammonium acetate | C2H7NO2 | 7.5M | 2.5M |
| Sodium chloride | NaCl | 5M | 0.3 to 0.5M |
| Lithium chloride | LiCl | 7.5M | 2.5M |
| Pottasium acetate | CH3CO2K | 3M | 0.3M |
Related articles:
- What is the Role of Alcohol in DNA extraction?
- Importance of Tris-EDTA (TE) Buffer in DNA Extraction.
- A Quick Guide on DNA Precipitation and Protocol.
Wrapping up:
Salt is an important ingredient in DNA extraction. It is not only used in precipitation but also for the preparation of lysis buffer and other buffer solutions. Precipitation of DNA is a simple process that needs an expert hand to make it effective.
Below-average expertise is enough for preparing DNA for routine PCR or hybridization, however, to deal with preparing DNA for sequencing and microarray, the chances of error are less. One has to have the utmost working hand.
The goal here is to prepare pure and high-yield DNA. In such conditions, precipitation becomes important. I hope you like this information. If so please share it.