“Setting up a genomics lab is a challenging process. Here are the 10 points that you should consider for the genomics lab setup.”
The genomic era is now!
I was at ThermoFisher’s reference lab (in Gurgaon, India) on Monday (26/2/2024) and we discussed how fast the genomic testing services are booming. They are selling more and more NGS machines every year.
Scientists, businesses, labs and organizations are getting interested in genomic services that include WGS, WES, transcriptomics, metagenomics, nutrigenomics and pharmacogenomic testing.
These services are costly, though provide lucrative data regarding a person or patient. Henceforth, genomics testing facilities are gaining more popularity. I thought why not cover a whole article on this?
Welcome to our comprehensive guide on setting up a genomics laboratory. The 10 points that I am explaining in this article are crucial information for those who want to open a genomics lab or want to upgrade their facilities.
I and my team have years of experience in setting up genetics and genomics laboratories. Besides, we remained constantly in contact with the related technology leaders to understand the technologies and their advancements.
Whatever information I am providing in this article is collected from my and my team’s industry knowledge and literature available on the Internet.
Let’s dive into the genomics.
Related article: How to Increase ROI for DNA Testing Labs?
Key Topics:
What is a Genomics Lab?
A genome is a complete set of DNA or a collection of an entire haploid set of chromosomes. Put simply, it’s a singular entire DNA of our cell. It possesses all the information for an organism to survive on Earth.
The human genome contains ~3.2 billion bases having coding genes, noncoding- introns, repetitive sequences, transposable elements, pseudogenes and other DNA sequences. Testing the genome means analyzing the entire genetic content of an organism.
A genomics lab is a specialized facility equipped with instruments that enable scientists to study a genome’s structure, functions, mutations and disease association. Scientists can study the entire genome sequence while also examining millions of alterations inherent within the genome.
This information certainly helps scientists to understand the nature of complex and polygenic conditions like cancer. For instance,
- They can identify genes and alterations associated with various types of health conditions.
- Identify disease-associated genes and genomic regions.
- Study larger and smaller copy number variations.
- Identify and study genome-wide SNPs and indels.
- Understand genomic instability associated with various cancers.
What are the technological requirements?
NGS- Next-Generation Sequencing is the main and prime technology requirement for the genomics lab, however, the microarray facility also provides substantial genomic information as well.
NGS provides sequence-based information regarding the genome. It can sequence the entire genome of an organism. To do so, various NGS chemistries such as short-read sequencing, long-read sequencing, single-end sequencing and paired-end sequencing are available.
Scientists have to choose the technology as per their requirements. The NGS platform is capable enough to handle many genomic operations, however, it’s a tedious, costly and time-consuming process.
Contrarily, microarray has been utilized to study known genomics variations including major and minor copy number variations and indels. It can help in analyzing thousands to millions of alterations, from the genome.
For setting up a genomics lab these two technologies must be required to study the whole genome of an organism.
What analysis can we do?
In a genomics lab, researchers can perform a wide range of analyses, including:
- Whole genome sequencing
- RNA sequencing (RNA-seq)
- DNA microarray analysis
- Comparative genomics
- Metagenomics
- Epigenomics
- Transcriptomics
(Links are given for each article, click and read the respective article.)
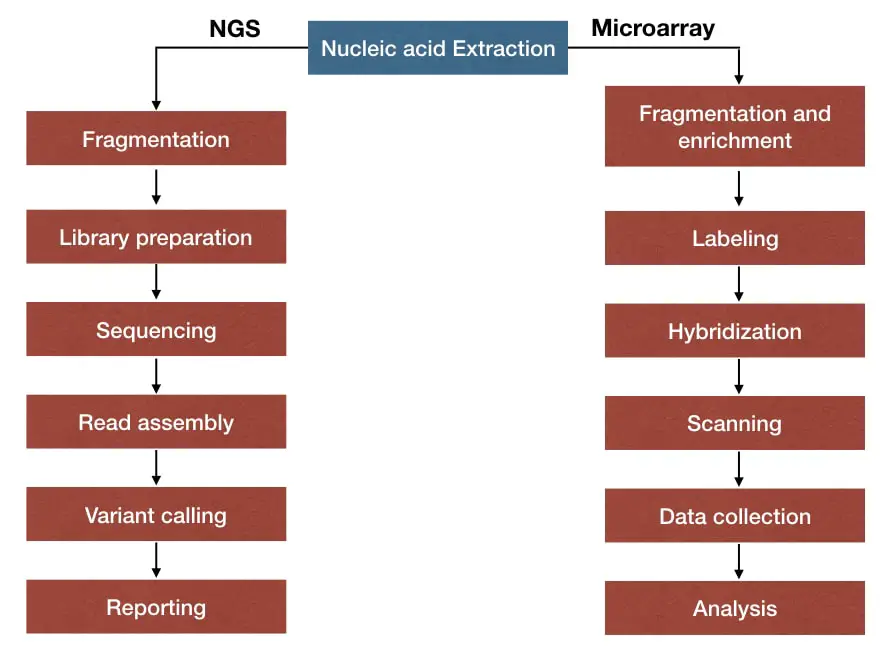
What is the workflow?
The workflow of a genomics lab typically involves steps like nucleic acid isolation, nucleic acid fragmentation, library preparation, sequencing or microarray analysis, data collection, and bioinformatics analysis.
The schematic representation of the NGS workflow and microarray workflow is given below.
Clinical vs Research Genomics lab
Clinical genomics labs focus on diagnostic testing, disease screening, and personalized medicine, while research genomics labs explore fundamental biological questions, genetic pathways, and evolutionary relationships.
Thus technological requirements are different for both. For clinical genomics, the lab has to perform disease or sample-specific analysis, deliver reports quickly, and perform highly accurate and sensitive analysis.
Thus they need a high throughput, sensitive, short read and robust sequencing platform having a wide range of ready-to-use assays available.
Contrarily, the research genomics lab has to collect comprehensive genomic data. They usually don’t need to deliver reports in time. However, they need to explore more genomic regions thus they should have to go for short and long-read sequencing platforms. Long read provides better sequencing accuracies for repetitive and complex genomic regions.
For service providers (nutrigenomics and pharmacogenomics) and clinical diagnosis, microarray should be required (along with NGS) but not necessary for research purposes. NGS can do the job better.
So it’s also important to know for a lab about their portfolio if they are going for research services or focusing on diagnosis and consumer services, and accordingly need to select the technology.
Genomics lab setup cost?
Do you know how costly the genomics lab setup is? The investment is in crores in INR. The basic setup may start from 8 to 10 crore and may need 50 to 60 crore in the future. Thus, running such a lab is certainly a challenge.
I always tell my clients that the genomics lab is a ‘Geneticist’s curse’ just like the airline business which is considered a ‘millionaire’s curse’. It’s a difficult thing to set up and run a profitable genomics lab.
Although experts like us (me and my team) can help to improve the ROI, still it needs keen planning, an expert brain in testing and run-planning and unique management skills.
For instance, suppose we have a sequencing chip with a capacity of 10 billion reads. We can do approximately 3 whole genome sequencing analyses (3.2 billion *3) and still, we have a half billion reads left in the chip.
We can add other samples, for example, breast cancer (BRCA panel) or even an exome sequencing sample to fill the chip. With this level of management skills, labs can generate profit.
This is just a simple example by the way. However, the geneticist or the lab personnel should know how to utilize the whole sequencing run whether it is a chip or cartridge for as many samples as possible.
Cost is the biggest challenging factor in setting up a genomics lab, but by doing such a micro-management ROI and break-even-point can be achieved fastly.
Bioinformatics infrastructure
Genomics is all about data. A machine will give us a huge file of gigabytes to terabytes in size with only nucleotide sequences. To access such a file and perform analysis, a robust bioinformatics infrastructure and an experienced team are required.
A genomics lab’s bioinformatics ecosystem should have high-performance computing (HPC) resources, bioinformatics pipelines, and data storage solutions for managing, analyzing and interpreting genomics data.
Computational infrastructure
In addition to the bioinformatics team and infrastructure, an excellent, sophisticated and state-of-the-art computational infrastructure is mandatory to perform analysis without abruptions and by ensuring scalability, efficiency, and reproducibility in genomic research.
The operation of such facilities demands high-end computer systems, including hardware, software, servers, and a stable Internet connection, alongside the implementation of robust data encryption and other IT solutions.
To ensure seamless operations, a dedicated team of IT experts is essential to oversee and maintain the computational infrastructure. Their responsibilities encompass constant monitoring to facilitate efficient bioinformatic analysis and address any technical challenges that may arise.
Ethical and Legal considerations
Personal information and data sharing are the two crucial and very opposite conditions a genomics lab has to face. Genomic data contains an individual’s sensitive and crucial information regarding their genetic makeup. Such information should remain confidential.
Contrarily, sharing the data with other labs, scientists or experts is essential for advancing scientific knowledge and accelerating genomic research. So there are serious ethical and legal issues involved in this case.
Ethical and legal considerations regarding patient privacy, informed consent, data sharing policy and compliance with regulatory standards such as the Health Insurance Portability and Accountability Act (HIPAA) are mandatory.
Here are some points to look after,
- Patient’s privacy and confidentiality will remain confidential.
- A fundamental ethical practice for informed consent should be followed.
- Patients should know about the data sharing policy (if any) while sensitive and confidential information sharing should be restricted.
- A genomic laboratory should follow national and international regulatory compliance.
- A patient’s data should be processed or used with integrity, honesty, and transparency.
Automation and throughput
In recent times, setting up a genomics lab isn’t that difficult, if you have a huge investment. However, running operations and generating the ROI is certainly a difficult task. And here you need an expert’s consultation.
Automation and throughput significantly influence your overall costs and ROI generation. Check out this image to understand this in a better way.
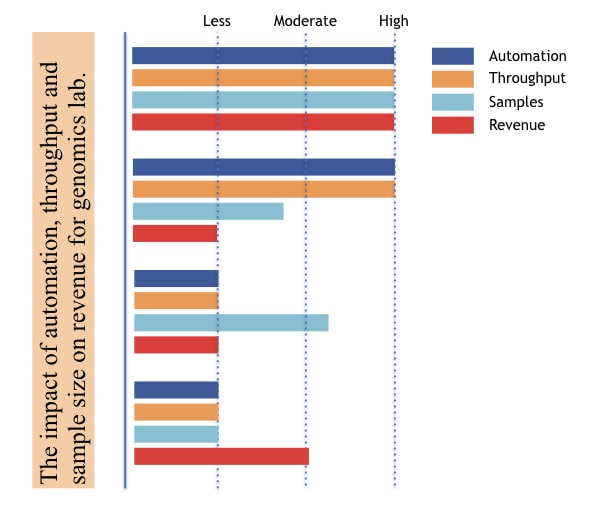
For example, if your lab has high automation, and high throughput but limited sample size, you end up in loss.
These two factors should be standardized with expert knowledge. A lab has to invest in automation and throughput as per their sample size or testing strength to improve their revenue.
Long term vision
A genomics lab should be guided by a long-term vision; success is not merely a matter of chance. By consistently expanding sample sizes and testing capacities, timely investments in automation and throughput can lead to a year-on-year improvement in ROI.
With the same facilities, you can achieve 5% to 50% ROI in a few years. We have a whole report on ROI improvement for the genomic testing lab, if you are interested let’s talk on a video call and discuss it.
Related article:
Wrapping up:
In conclusion, setting up a genomics lab requires precise planning, huge investment, and commitment to scientific excellence. By understanding the requirements, workflow, costs, ethical considerations, and long-term vision, one can set up a profitable genomics lab.
We hope this guide has provided valuable knowledge regarding establishing a genomics lab and will inspire you to set up your own. If you have any plans to start your lab or want to upgrade your existing genetic lab with genomic facilities, do contact us.
I and my team will help you in your journey.
Email us: [email protected] or [email protected]