The ‘group of muscle disorders’ associated with muscle weakness and breakdown due to some genetic factors are known as Muscular Dystrophy.
Genetics factors such as either gene mutations, chromosomal alterations or both are predominantly involved in various types of disorders, those are categorised under genetic disorders.
Usually, genetic disorders are inherited or transmitted from parents to their offspring, Obey one of the following inheritance patterns; autosomal dominant, autosomal recessive, X-linked dominant, X-linked recessive or Y- linked.
muscular dystrophy is also a class of genetic disorder following one of the inheritance patterns listed above. Muscle weakness, movement trouble and muscle breakdown are some common symptoms observed in all types of dystrophy.
30 different muscular dystrophies are categorised till date amid Duchenne muscular dystrophy (DMD), Becker muscular dystrophy (BMD), limb-girdle muscular dystrophy, myotonic muscular dystrophy and facioscapulohumeral muscular dystrophy are some most common types of it.
Currently, no cure for any type of muscular dystrophy is available. Gene therapies like CRISPR-CAS9, exon skipping and insertion of mutations can be helpful in future.
In the present article, we will discuss one of the most common types of genetic disorder- muscular dystrophy. Also, we will talk about various types and genetic mechanisms involved in it. As we said, we will discuss gene therapy treatment in the last segment of this article.
So let us dive into the topic,
Key Topics:
What is muscular dystrophy?
The MD, abbreviated as “muscular dystrophy” is a genetic disorder associated with the muscle or abnormal behaviours of various bodily muscles.
In 1830, Charles Bell had described the present genetic condition in a muscularly weak boy. Guillaume Benjamin Amand Duchenne, a French neurologist was the first person who described conditions of Duchenne Muscular Dystrophy during 1861. From his name, it is known as Duchenne muscular dystrophy.
After the genetic basis of muscular dystrophy was established by William Richard Gowers; Louis M. Kunkel had given the molecular mechanism and explained the X-linked inheritance of the DMD condition in 1986.
Interestingly the word was originally derived from the Greek word ‘dys’ means ‘difficult’ and ‘trophy’ means ‘nourish’
Definition:
A type of genetic condition, caused by various gene mutations and associated with muscular deformation and weakness is categorised into a group of “muscular dystrophy”.
Sign and symptoms:
- Muscle weakness
- Progressive wasting of muscle
- Break down of muscle
- Balancing problem
- Movement and walking problems
- Calf deformation
- Waddling gait
- Muscular stiffness and pain
- Several learning difficulties
- Respiratory difficulties
- Muscle spasms
- Cardiomyopathy
- Difficulty in swallowing
- Standing and sitting difficulties
- Poor muscles
These are some common problems and symptoms associated with dystrophy. Now after understanding the symptoms we need to understand the cause of it.
What causes muscular dystrophy?
The present disorder is a kind of genetic condition or disorder. Mutations in genes located on the X chromosome or autosomes are responsible for it. X-linked dominant, X-recessive, autosomal dominant or autosomal recessive pattern of inheritance is observed in the case of various types of muscular dystrophy. However, not all muscular dystrophies are inherited.
The Dystrophin protein present in bodily muscle cells gives strength, spring-like shock absorbance mechanism to muscles. Broadly, the protein dystrophin functions for repairing and constructing muscles.
Every different muscular dystrophy occurs due to various mutations in different genes involved in the formation and functioning of dystrophin. Although it is a complex group of protein, they help in proper muscle working.
Mutations in genes associated with dystrophin hinder in formation or disrupt its normal function, or in some cases make the entire protein inactive. The dystrophin can’t work properly in these cases and creates different muscle-related problems.
As the present condition is X-linked (mostly), the chance of disease occurrence is high in males compared to females. Just because male have only a single X-chromosome, even if the inheritance pattern is X-linked dominant or recessive, it doesn’t matter. They always remain affected.
As we said above, not all types of muscular dystrophy are inherited, due to replication error and other spontaneous mutations during the developmental stage, various muscular dystrophy can be originated.
Notably, the effect of the mutation depends on the expression of the dystrophin in various muscle cells.
Types of muscular dystrophy:
- Duchenne
- Becker’s
- Myotonic
- Limb-girdle
- Oculopharyngeal
- Facioscapulohumeral
- Congenital
- Distal
- Emery-Dreifuss
Duchenne muscular dystrophy:
The DMD- Duchenne muscular dystrophy is an X-linked recessive form of the genetic disorder. Mutation or mutations in the gene DMD causes abnormal or non-functional dystrophin protein. Cytologically, the gene is located on the X chromosome between Xp21.2 to Xp21.1.
The main function of the dystrophin as we said it to repair and give strength to muscle fibres. The protein is chiefly located in the skeletal, chemical cell signalling, cardiac and other vital muscles, abnormal function of it causes various muscular problems.
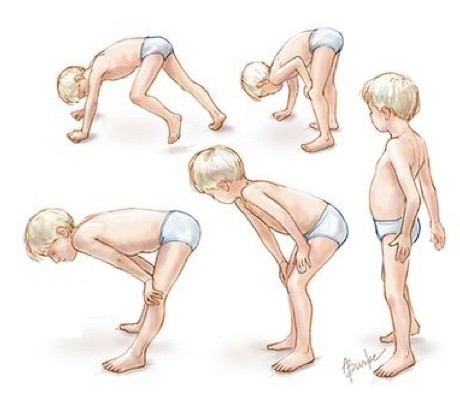
The structure or function of protein dystrophin altered by mutations in DMD gene results in muscle weakness, muscle loss and abnormal movement.
The condition is known as progressive muscle weakness or muscle wasting, also known as atrophy. Interestingly, it is also observed in Becker’s muscular dystrophy as well.
In males, one single mutant copy of a gene is sufficient to cause the disease because of having only a single X-chromosome. On the other side, in females, the chance of disease is very less (because they have two X chromosomes). However, females remain carriers for the disease.
Both Becker and Duchenne muscular dystrophy affect 1 in 5000 newborn male worldwide.
Cardiomyopathy is ordinarily observed in both types of muscular dystrophy predominantly. In some rare cases, problems like muscle weakness and cramps are observed in carrier females as well. Usually, carrier females remain unaffected and don’t show any signs and symptoms of the disease.
Notably, the DMD is more common in children under the age of 2 to 6. They are unable to walk properly and need a wheelchair.
Becker’s muscular dystrophy:
The same gene which is responsible for Duchenne muscular dystrophy is the reason for the present genetic condition. Therefore, the sign & symptoms, inheritance pattern, chromosomal location and causes are the same as the above condition.
Myotonic muscular dystrophy:
The Myotonic muscular dystrophy often called myotonic dystrophy are of two types, the type 1 MMD is occurring due to DMPK gene mutations while type 2 occurred due to CNBP gene mutations.
The DMPK gene is located on chromosome 19 at 19q13.32 while the CNBP gene is located on chromosome 3q21.3. Interestingly, both types of myotonic dystrophy are inherited in an autosomal dominant manner.
Only a single copy of a mutated gene in both male and female is enough to cause disease.
The mechanism is called ‘anticipation’ in which symptoms of the disease become more severe as the age increases observed in present condition. Anticipation is more distinct and clear in case of myotonic dystrophy type 1, although.
Progressive muscle weakness, breakdown and wasting are reported in this condition, however, the symptoms begin in adulthood. Unrelaxed muscle contraction- myotonia with slurry speech and temporary jaw locking commonly appear.
Furthermore, other muscular and cardiovascular problems are seen in the patients.
Congenital myotonic dystrophy occurs by birth is actually a type 1 MTD. Signs and symptoms of the disease appear by birth. Clubfoot, breathing problem, hypotonia and some intellectual and developmental disabilities are observed in congenital type 1 MTD.
Broadly we can say, both the type of myotonic dystrophy affects 1 in 8000 people worldwide.
Limb-girdle muscular dystrophy:
As the name suggests, in the present dystrophy condition, arms’ and legs’ muscle wasting is ordinarily observed. Limb-girdle muscular dystrophy is a polygenic disorder in which various different autosomal genes are involved.
Here both autosomal dominant and autosomal recessive inheritance patterns are observed in various types of limb-girdle dystrophy. The muscle abnormalities of legs and arms such as shoulders, pelvic area, thighs and upper & lower arm seen in patients.
Several genes their location and type of Limb-girdle muscular dystrophy are given into the table below,
| Sr no. | Gene | Location | Type of LGMD |
| 1 | CAPN3 | 15q15.1 | 2A |
| 2 | DYSF | 2p13.2 | 2B |
| 3 | SGCA | 17q21.33 | 2D |
| 4 | SGCB | 4q12 | 2E |
| 5 | SGCG | 13q12.12 | 2C |
| 6 | SGCD | 5q33.2-q33.3 | 2F |
| 7 | TNN | 2q31.2 | 2J |
| 8 | ANO5 | 11p14.3 | 2L |
Oculopharyngeal muscular dystrophy:
The OPMD is caused by gene mutations of the PABPN1 gene, located on chromosome 14, 14q11.2 to q13. It is a type of autosomal dominant form of genetic disorders most commonly observed in France and French people. In France 1 in 10,000 newborns are affected by the disease.
The OPMD follows an ‘anticipation’ mechanism in which the disease symptoms are observed in adulthood or after 40 years of age. Swallowing difficulties and dropping of eyelids are two common symptoms seen in the patients, however, the symptoms vary among patients.
Like other muscular dystrophies, muscle weakness and muscle wasting are usually examined in the present genetic condition as well.
Facioscapulohumeral muscular dystrophy:
The muscles of the face, around the shoulder blades and upper arms (facio- scapulo- humeral-) are commonly affected in this condition. Hypomethylation in the region D4Z4 located on the long q arm of chromosome 4 causes FSHD1.
Usually, this region has 11 to 100 repeat sequences, however, in the disease condition, the D4Z4 region becomes shortened up to 10 repeats.
Mutations in other genes such as SMCHD1and DUX4 also result in present genetic conditions. Those cases are few.
Plenty of muscular weakness problems are associated with the present condition, like the eye can not fully close when a person sleeps, difficult to drink and smile.
As the age increases, the symptoms become more worsen, strong and can spread to other body parts.
Congenital muscular dystrophy:
A heterogeneous disease group with autosomal recessive inheritance pattern occurs by birth known as congenital muscular dystrophy. Unlike other muscular dystrophies (myotonic or Facioscapulohumeral) in which the disease symptoms appear late onset of age (in or after adulthood), in the congenital muscular dystrophy, the disease symptoms occur by birth.
The gene for the CMD is LAMA-2 and is located on chromosome 6 at 6q2.0. It occurs 2 to 4 in 10,000 newborns worldwide.
Progressive muscle weakness in various body parts and atrophy is observed in patients likewise. Sitting, standing, rolling and walking problems are predominantly observed in affected children. Congenital muscular dystrophy is a more severe type of condition, as age increases, the disease becomes worse.
Distal muscular dystrophy:
Distal muscular dystrophy occurs in the muscles far from the body centre like the muscles of the lower arm, legs, hands and feet. Muscle wasting and breakdown observed in the present condition as well.
It affects only several types of muscles, thus the disease progression is slower and mild. Various genes are involved in various type of distal muscular dystrophy, those are listed here:
| Distal MD | Gene | Location |
| Laing distal myopathy | MYH7 | 14q12 |
| Udd distal myopathy | TNN | 2q24.3 |
| Inclusion body myopathy type 2 | GNE | 9p12-p11 |
| Miyoshi myopathy | DYSF | 2p13.3-p133.1 |
As the present genetic condition is polygenic, the clear inheritance pattern of the disease is unclear, some follow either autosomal dominant or recessive inheritance patterns.
The distal muscular dystrophy is caused by autosomal genes, therefore, the chance affected male and female are equal.
Emery-Dreifuss muscular dystrophy:
In 1960, Alan Emery and Fritz Dreifuss described the present type of dystrophy which affects the bodily voluntary muscles.
Emery-Dreifuss muscular dystrophy is a polygenic disorder, genes like EMD, FHL1 and LMNA are involved in various types of Emery-Dreifuss myotonic dystrophy.
Various genes, their location and inheritance pattern are enlisted in the table below,
| Gene | Chromosome | Location | Inheritance pattern |
| EMD | X chromosome | Xq28 | X-linked recessive |
| FHL1 | X chromosome | Xq26.3 | X-linked recessive |
| LMNA | Chromosome 1 | 1q22 | Autosomal dominant |
Notably, in some rare cases, an autosomal recessive inheritance of the LMNA gene is observed, however, those cases are very few. Other genes like SYNE1, SYNE2 and TMEM43 also cause other types of EDMD, although those cases are very rare.
The commonest symptoms of the present muscular dystrophy observed in the majority of patients is the conduction block or blockage in the heart besides muscle wasting and weakness.
Furthermore, stiff joints in neck, elbows and heels during the early onset of the disease is commonly found. Anticipation also happens in EDMD, as the age of the patient increases, the disease becomes worse, the muscle problems spread to the other body parts. Cardiovascular problems are usually observed in adulthood.
Other symptoms are,
- Palpation (pounding in the chest)
- Bradycardia (slower heartbeat)
- Arrhythmias (unsync heart rhythms)
- Syncope (fainting)
The main reason or cause for the dystrophy here is the non-functioning or dysfunctioning of various genes involved in the present condition.
Diagnosis:
For different types of muscular dystrophy, various diagnostic methods are available such as muscular biopsy, Electromyography, Nerve conduction study, cardiac examination, Enzyme assay and genetic testing. Amid all, the genetic testing methods are most trusted, accurate and reliable methods.
PCR test, DNA sequencing and comparative genomic hybridization are routinely used in genetic or DNA test for muscular dystrophy diagnosis.
PCR test: A known mutation or mutations can be identified using the polymerase chain reaction. In the PCR, gene amplification helps to find out mutations. However, the present method is reliable only for single-gene disorders. Further, larger genes like the DMD can’t be amplified entirely.
DNA sequencing: The present testing method is even more accurate and reliable than the PCR based detection method. Here, an entire gene or genomic region is amplified and identified.
Sequencing identifies every single nucleotide of a gene, therefore, new various or mutations can also be encountered here. The principle of DNA sequencing is simple, the fluorescently labelled probe or dNTPs binding to the growing DNA strand and emits fluorescence signals which are recorded by the machine.
Through the computational analysis, the gene is compared with available DNA sequencing, to find out any new various.
The sequencing method is powerful enough to find out even a single base variation in a gene. Because of this reason, it is more preferred.
Comparative genomic hybridization: Comparative genomic hybridization method is able to identify a large number of mutations from various genes in a single assay. Furthermore, It can measure gene expression level as well.
Mutant variant-specific oligo sequences are immobilized on the solid glass surface when a sample is applied on it, a complementary sequence of various genes binds to those oligo sequences and provides various hybridization signals.
Although the specificity and sensitivity of the assay are lower than the DNA sequencing method. But due to its capacity to find out thousands of various mutations in a single assay, it has more advantages than other genetic testing methods.
High cost, time-consuming sample preparation and data interpretation make it disadvantageous.
Conclusively, muscular dystrophy is a type of genetic disorder, genetic testing or DNA test used so often to encounter or find out tentative reasons behind any type of dystrophy.
The genetic testing method is more reliable, reproducible, trusted, and effective, at the same time, these methods are time-consuming, expensive and laborious.
Other testing methods:
Electromyography: Muscular electrical activity helps to find out muscle and related abnormalities.
Biopsy: A biopsy- a portion of a muscle is taken and examined under a sophisticated microscope to detect muscle problems or myotonia.
Heart monitoring or cardiac monitoring: As the cardiac muscles are affected more in the muscular dystrophy, especially in the DMD, using electrocardiograms or echocardiograms, the muscular activity of the heart is monitored.
Lung function or monitoring: checking the function or activity of lung muscle can also give some information about the condition.
Protein assays: Using several immunological and chromatography techniques, protein abnormality and protein activity can be determined. Also, a mutant protein can be identified and characterised.
Enzyme assay: The activity of several enzymes involved in the muscle activity and muscle function can be measured and by doing so, the activity of various enzymes can be determined.
Treatments:
No approved cure or medications are available to recover any type of muscular dystrophy. However, using several medications, therapies and exercise, the disease progression and severity can be minimized.
By doing some basic exercise and stretching, muscle strengthening can be done. Sometimes breathing assistance, braces and mobility aids like canes, wheelchairs and walkers are required to stay mobile.
Nonetheless, the doctor’s advice should be taken before doing any exercise and taking any medications.
Yoga will help patients to stay strong for a longer period of time. Take advice from the yoga expert and do yoga which strengthens overall muscular activity.
Gene therapy for muscular dystrophy:
It is true that no cure till now is available for any type of muscular dystrophy, however, there are several gene therapy options that can help to repair some.
Using gene therapies, a faulty gene can be removed, repaired or replaced by another wild type one. It is the most promising and reliable option at least for now to treat genetic disorders by targeting genes.
A known vector transfers a gene of interest to the target location or in a gene through gene transfer. CRISPR-CAS9-like tools can remove faulty DNA sequences. Here in this section, I am enlisting some of the ongoing gene therapy experiments that are used in the treatment of various types of muscular dystrophies.
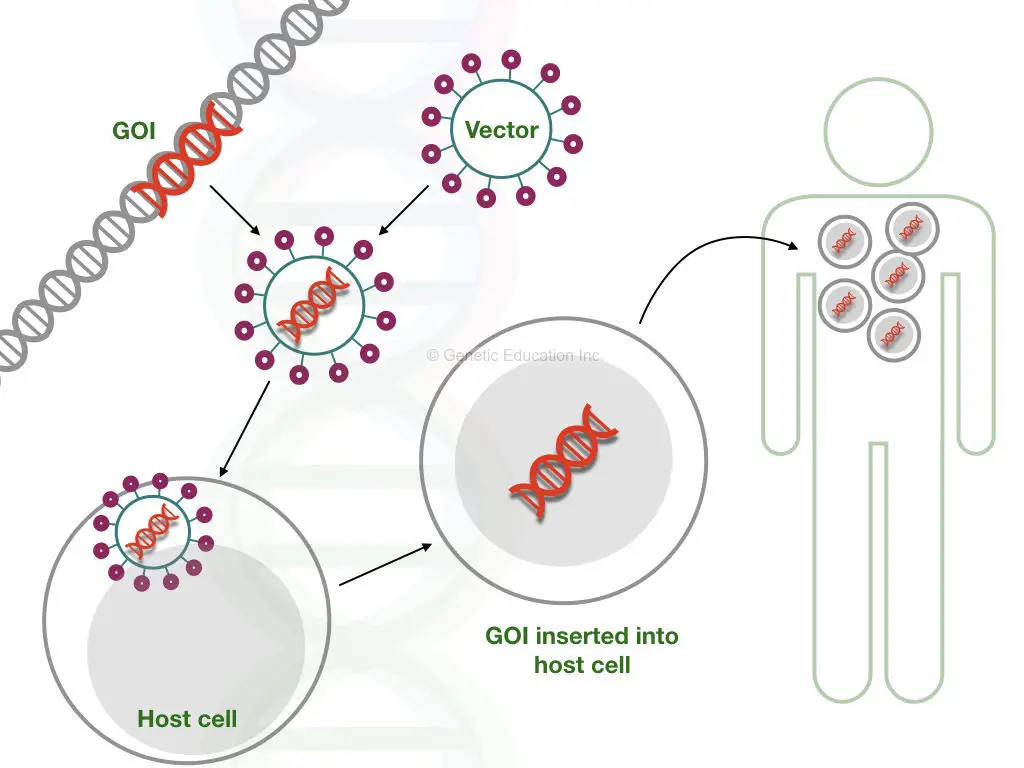
CRISPR-CAS9:
The present gene therapy is one of the most promising and successful options amid all types of gene therapies. The CAS-9 is a nuclease protein that cleaves DNA at specific locations using the CRISPR system.
Due to its promising results, it is being continuously investigated for DMD. A normal function of the dystrophin gene can be restored by removing the faulty gene. Scientists have reported some positive results during pre-clinical trials.
Exon skipping:
Exons are coding regions of a gene, during gene expression, the introns or non-coding sequences are removed from the final transcript and only exons are left in a gene.
By exon skipping, a faulty, non-functional or inactive exon or exons of the dystrophin gene are removed using antisense oligos. Although it leads to truncated or incomplete protein formation, it helps to ease some of the symptoms.
Exon skipping can’t repair the entire gene however, some function can be restored.
The present therapy is being approached especially for DMD and BMD. The FDA approved Exondys 51 is now commercially available for DMD.
Inserting stop codon in a dystrophin gene is yet another promising gene therapy approach. In this technique, a stop codon is inserted to terminate translation prematurely. Fully mutant dystrophin protein can’t be formed.
The largest gene dystrophin can’t be entirely removed, however, using AAV (Adeno-associated virus) mediated gene transfer technique, a mutated portion of a gene can be replaced.
also, a mutant GALGT2 gene can be replaced with a healthy one. Some of the AAV-vector mediated gene therapies are now under the final phase of a clinical trial.
Undoubtedly, gene therapies are the most promising! It may cause adverse or side effects, the normal function of a gene can’t always be restored. Also, it can’t work every time.
Conclusion:
The muscular dystrophy is a condition of muscle weakness, breakdown and degeneration. It has a distinct inheritance pattern and follows the mechanism of anticipation, in most cases.
Various genes are involved in different conditions, due to the polygenic nature of some dystrophies, it is very difficult to find the exact reason behind it. Furthermore, not all mutations are to date discovered.



Good notes sir. It’s very useful to us
Thank you Dr Nisha