“Learn about the basics of Phenol: Chloroform DNA extraction including chemical preparation and step-by-step protocol. This PCI DNA extraction guide from Genetic Education provides valuable insight for researchers and students alike, covering safety precautions and optimization tips, everything.”
The phenol-chloroform DNA extraction method is commonly used in molecular genetics and is capable enough to isolate high-quality and high-yield DNA from any biological sample. Other names of this technique are phenol DNA extraction, phenol-chloroform, phenol-chloroform isoamyl alcohol DNA extraction or PCI isolation method.
Among all the different types of DNA extraction methods, the present method is popular among students as well as seasoned scientists too. Three organic solvents it uses are phenol, chloroform and isoamyl alcohol.
PCI DNA isolation technique can extract DNA from any cell or tissue type including mammalian cells, plant parts, bacteria or even plasmids. But the technique is complicated, time-consuming and potentially hazardous.
At Genetic Education, our main objective is to re-research and present knowledge that is easy to understand and useful. I and my team have years of experience working on DNA extraction, electrophoresis and PCR.
So we are the best at explaining these concepts.
In this article, I will explain the basics of PCI DNA extraction and explain the stepwise process and protocol. This comprehensive guide will also demonstrate the advantages, limitations and optimization tips.
Stay tuned.
Check out our product to learn more about DNA extraction, electrophoresis and PCR.
Key Topics:
Principle of PCI method:
As we said earlier, phenol-chloroform isoamyl alcohol relies on the principle of liquid-liquid extraction of biomolecules. It denatures the protein part and separates the genomic DNA into a soluble phase.
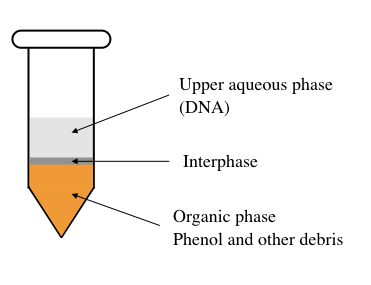
To understand it precisely, we need to look inside the tube, let dive into the tube.
Suppose the tube is filled with phenol, chloroform, isoamyl alcohol and cell suspension. The phenol is less-polar while the watery part (containing chloroform) is polar in nature. Also, note that phenol is denser than water so remained at the bottom of the tube.
DNA is a polar molecule having a negative charge. The principle of the polarity of biomolecules says that the polar molecules dissolve in the polar solvent and the non-polar molecules in the non-polar solvent.
Henceforth, water (present in the solution) dissolve DNA but not protein while phenol can’t dissolve the DNA. Due to the higher density of phenol, it remains at the bottom. So the genomic DNA remains in the upper watery-soluble part while the cell debris remains at the bottom.
Centrifugation settles cell debris and protein in the lower phenolic phase whilst the nucleic acid can be collected carefully from the upper phase.
This is the simplest explanation of the principle. Importantly during the process, emulsification happens, meaning a foam-like emulsion forms which should be removed first. Note this point, I will explain this part (how to remove foam) later.
Role of each chemical:
The technique becomes more aggressive when the isoamyl alcohol is used along with phenol and chloroform therefore the technique is often known as PCI DNA extraction. The in-depth role of three major constituents is explained here.
Phenol:
DNA is insoluble in phenol because phenol is a less-polar solution.
On the other side, protein has both polar and non-polar groups because of the long chain of different amino acids. The amino acid side chains have varied groups.
Also, the folding of the protein into the secondary, tertiary and quaternary structure relies on the polarity of the amino acids. When we add phenol, bonds between amino acids break leading to protein denaturation.
We can say, phenol unfolds the protein structure and digests it.
Chloroform:
The main function of chloroform is to protect genomic DNA during a catastrophe.
Chloroform increases the efficiency of phenol to denature the protein. Here, chloroform allows proper separation of the organic phase and aqueous phase and keeps DNA protected into the aqueous phase.
Note that, chloroform denatures the lipid as well.
Isoamyl alcohol:
Remember the foaming during phenol mixing?
In the phenol-chloroform DNA extraction method, Isoamyl alcohol helps in reducing foaming between interphase. It prevents the emulsification of a solution.
The liquid phase contains DNA and the organic phase contains lipids, proteins and other impurities. The precipitated protein denatures and coagulates between both these phases. This will create a cloudy, whitish- foam between interphase.
The anti-foaming agent, isoamyl alcohol stabilized the interphase by removing the foaming and increasing the purity of DNA. Noteworthy, the isoamyl alcohol is also practiced as a DNA precipitation agent in the later stage of the extraction process. Briefly, the role of other chemicals is explained in the table below,
|
Chemical |
Role in DNA extraction |
|
Tris |
It maintains the pH of the solution and also permeabilizes the cell membrane. |
|
EDTA |
It is a chelating agent and blocks the activity of the DNase enzyme. |
|
SDS |
It is an anionic detergent that helps in the denaturation of cell membrane protein. |
|
NaCl |
Prevents the DNA denaturation |
|
MgCl2 |
Protects DNA from mixing with other cell organelles |
|
TE buffer | Dissolves DNA |
Preparation of phenol:
We cannot use phenol directly, we have to prepare saturated phenol before proceeding further. The commercially available phenol comes in crystalline form, we have to saturate it before use.
I have performed many DNA extractions and prepared phenol a thousand times. Here is my protocol to prepare the saturated phenol and you can use it.
Saturation of phenol:
Take the bottle of phenol from the deep freezer and put it at room temperate for some time. After that put the bottle of phenol in the 65°C water bath.
Thaw it at 65°C until the phenol becomes liquid.
Now take the required amount of phenol into a flask and add 0.1% W/V 8- hydroxyquinoline.
Add an equal volume of 0.5M Tris-HCl at pH 8.0
Put the flask of phenol on the magnetic stirrer for 20 to 25 minutes. Stir it and mix well.
Transfer the mixture of phenol and Tris HCl into the separating funnel and leave it for 10 to 12 hours for separation.
After 12 hours we get the organic phase and aqueous phase. Collect the lower phase (organic phenol phase) and check the pH with a pH strip. Set the pH between 7.8 to 8.0.
Repeat all the steps until you get the phenol with a pH of 7.0 to 7.5. From the second cycle onward use 0.1M Tris instead of O.5M Tris.
After completion of phenol equilibration, collect phenol in a red amber bottle and add a pinch of 0.2% W/V beta-mercaptoethanol.
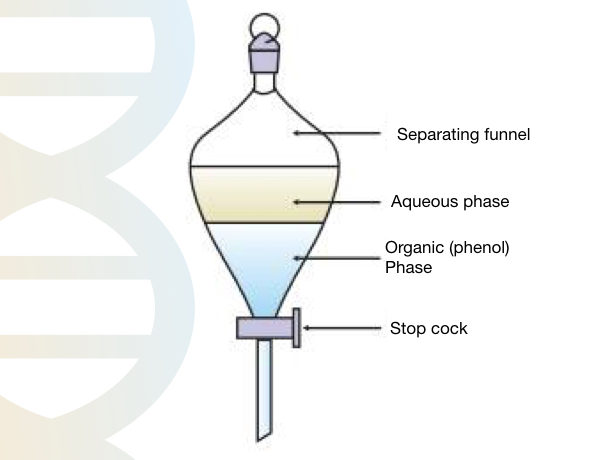
Overlay 1 cm of 0.1M Tris into the bottle to protect the phenol (it is light sensitive) and store it at 4°C. Don’t use phenol if pH is changed.
During phenol preparation wear gloves and an eye protector, don’t expose phenol to sunlight. Phenol is volatile and can burn your skin, so handle it carefully.
The preparation of phenol is an important step in DNA extraction, if you prepared phenol well, you will get good results. Soon after, we need to prepare a solution of phenol: chloroform: isoamyl alcohol.
Preparation of phenol-chloroform isoamyl alcohol
The magic of PCI DNA isolation relies on the effective chemical composition, meaning, how and in which amount you use the three ingredients. You will get excellent results if all ingredients are correctly weighed and used.
The concentration of chloroform and isoamyl alcohol is as important as phenol. The phenol can be used in combination with chloroform and isoamyl alcohol in three different steps,
- In the very first step use, only phenol
- In the next step use phenol: chloroform: isoamyl alcohol (25: 24:1)
- In the last step use Chloroform: isoamyl alcohol (24:1)
For 25: 24:1 preparation of PCI, take 25 ml of phenol, 24 ml of chloroform and 1 ml of isoamyl alcohol and mix it well. For 24:1 chloroform: isoamyl alcohol adds 48 ml of chloroform and 2 ml of isoamyl alcohol to prepare 50 ml solution.
Note that to achieve excellent results prepare each solution fresh every time when doing DNA extraction. Also, prepare it as per your requirement, if you need 10 ml, weigh each ingredient accordingly.

Protocol for Phenol-chloroform and isoamyl alcohol:
The detailed protocol is explained here and this is one of the best standard protocols from our lab.
Collect 5 ml of blood and add 5 ml of solution-I (equal volume) and add 120 µl of Nonidet P40, gently mix by inverting several times until Nonidet P40 is mixed in the solution, centrifuge at 2000 rpm for 20 min.
Discard the supernatant and add 800 µl of solution-II, the sample should be handled gently.
Transfer it to a 2 ml Eppendorf tube, now add 400 µl saturated phenol, mix well and centrifuge at 12000rpm for 1 min.
Take supernatant and add 800 µl of phenol: chloroform: isoamyl alcohol (25:24:1) (400 µl saturated phenol: 384 µl chloroform: 16 µl isoamyl alcohol ) mix well and centrifuge at 12000rpm for 1min.
Take supernatant and add 700 µl of chloroform: isoamyl alcohol (24:1) (672µl Chloroform: 28µl Isoamyl alcohol), mix well and centrifuge at 12000 rpm for 1 min.
Take supernatant and add a double volume of chilled ethanol or add 1/10 vol. of sodium acetate and an equal volume of isopropanol (chilled).
Mix well by inverting until DNA precipitate appears.
Centrifuge at 12000rpm, remove the supernatant and wash DNA with 70% ethanol. Minimum 2 and maximum 5 washes should be given to DNA so that we can get pure DNA.
After the final wash discards the alcohol and dries the pellet overnight or in the hot air oven for 15 min at 50°C.
Add 100 to 500 µl of dd/w or TE buffer depending upon the quantity of pellet. Now transfer the Eppendorf tube to the water bath at 65 to 70°C temperature for 30 min or until DNA dissolves.
The given protocol is for 5 ml of a blood sample and it is standardized by our team. You can use it directly. Also, you can modify it as per your sample quantity.
Advantages:
- One of the most trusted, well-known and widely accepted methods of DNA extraction is our PCI method.
- We get good DNA purity and yields.
- The present method is cheap, easy to use and reliable.
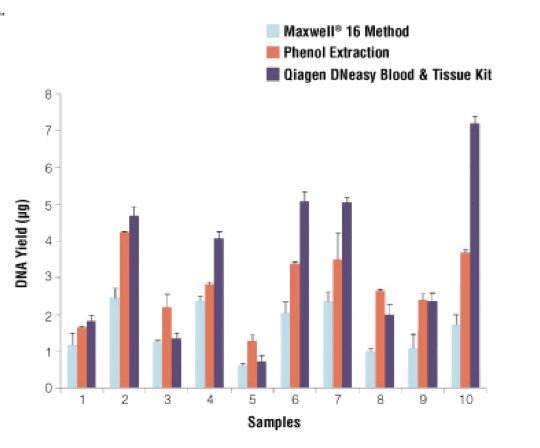
Stephanie Bougel and Jean Benhatter used different methods for extracting DNA from 10 unrelated samples. As shown in the graph, Among all 10 samples, the PCI gives a higher yield of DNA in comparison with the Maxwell 16 method.
However, the yield is lower as compared with Qiagen DNeasy Blood and tissue kit. Still, the amount of DNA obtained from the phenol-chloroform DNA extraction method is good. To read the full research paper of Stephanie Bougel and Jean Benhatter, click here.
Limitations:
- The phenol-chloroform method of DNA extraction is time-consuming and tedious. However, by standardizing it properly, we can use it routinely.
- Also, the process of chemical preparation is time-consuming and tedious too.
- The chemicals used in phenol-chloroform DNA extraction are dangerous for us which is the major limitation of the PCI method.
- The phenol is volatile and may cause skin burns and irritation.
- The chloroform makes you unconscious.
- Moreover, what you get depends on how you work, meaning if you don’t have a good practical hand, you can’t isolate good DNA.
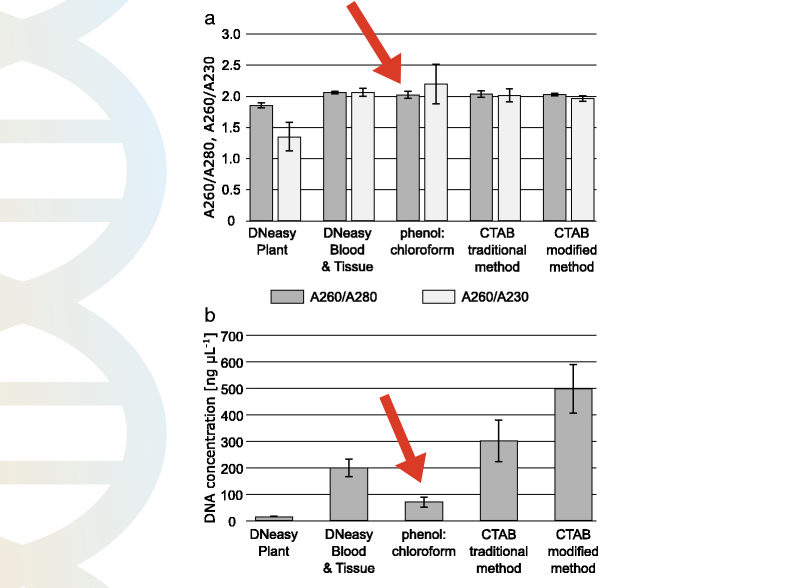
Natalia Gumińska et al. used the DNeasy Plant DNA extraction kit, DNeasy Blood and Tissue DNA extraction kit, phenol-chloroform method, and CTAB method to extract DNA from various sources.
As per their findings, the purity and quantity obtained from the phenol-chloroform DNA extraction method were very less. The 260/280 ratio of the PCI method wasn’t so good and the quantity too not so sufficient.
The graphical representation of their findings is shown in the figure above. You can read their article here, click here.
In summary, the purity and quantity obtained by the phenol-chloroform isoamyl method aren’t so good.
My suggestions:
Safety is a priority, chemicals used during DNA extraction can be harmful in many ways therefore always wear gloves, an eye protector, a head cap and a face mask.
While handling phenol, always wear a lab coat and eye protector because phenol is volatile and can burn your skin. It can also damage our eyes hence do not compromise safety.
The chloroform can make you faint or unconscious, a high dose can be lethal so take enough precautionary steps before performing.
Moreover, we need to protect our chemicals and solutions too. For instance, phenol can oxidize when exposed to sunlight therefore always store phenol in a dark or amber bottle.
Also, the pH can be fluctuated at a higher temperature so always store phenol at 4°C, and check the pH periodically.
Prepare fresh phenol: chloroform: isoamyl alcohol every time before DNA extraction.
Buy Our ebook:

Conclusion:
For a startup or a new lab, the present method (Phenol-chloroform and isoamyl alcohol DNA extraction) is the best option- cheap, effective and reliable. It cuts costs and gives excellent results too if performed well.
I personally believe that every student should learn manual DNA extraction, the use of chemicals, solution preparation and protocol standardization. Although ready-to-use kits are now common, try to learn manual things, thrust me it makes your practical hand more efficient.
Always prefer to do DNA extraction with your own combination of chemicals, it will boost your knowledge and confidence.


DNA pellet comes in green and black color as well as in large amount. how to resolve it
amazing blog. Being a student I totally rely on this
Thank you Naman
Please site the reference for this method.
Much usefull.God bless You.buddy.keep it up
i have prepare the mixture of phenol cgloroform and isoamylalcohal in 24: 24 :1 ratio but after mixing phenol into chloroform isoamyl alcohal solution it forms 2 phases seperately. can you please guide ?
This site is simply superub………
Thank you so much Nadeer.
Excellent research on DNA Extraction method..
Well done team..l love this site.
Thank you Ashish Anant
Great blog!! That’s very useful information in this my life, great research on this topic.
Thank you alex