“Ethidium Bromide (EtBr) is a DNA-intercalating fluorescent dye used to visualize DNA during gel electrophoresis. Explore why and how we use it in electrophoresis.”
Electrophoresis is a technique to study the DNA or RNA, in short, nucleic acid. Unfortunately, either nucleic acid is colorless and thus can’t be tracked and visualized.
In the previous article, I explained how we can track DNA migration using the gel-loading dye. It helps us to monitor and control the migration in a gel but not in visualization. To study the DNA, we need to add some colored substance that can bind with DNA and give us the strength to visualize it.
A fluorescent dye Ethidium bromide allows us to study DNA under UV light. How? Let’s find out. In this article, I will explain why and how we use EtBr in Gel electrophoresis, its properties, concentration and disposal guidelines.
Stay tuned.
Key Topics:
What is Ethidium bromide?
Ethidium bromide, short for EtBr/ etbr or EB, is a non-radioactive, fluorescent and intercalating dye. It is an aromatic heterocyclic compound. The chemical name of EtBr is 3, 8-Diamino-5-ethyl-6-phenylphenanthridinium.
Two other names are Homidium bromide or chloride salt homidium chloride. It’s a red-orange fluorescence dye that can emit fluorescence when exposed to UV light. It is non-flammable but carcinogenic in nature. Thus care must be taken while handling it.
| Chemical | Ethidium bromide |
| Abbreviations | EtBr, etbr or EB |
| Chemical formula | C21H20N3Br |
| Molecular weight | 394.4 |
| Fluorescence | Orange |
| Toxic | Yes |
| Appearance | Dark red |
| Availability | Crystalline form |
| Odour | odorless |
| Property | DNA intercalating agent |
Interestingly, during the 19th century, it was used as an anti-trypanosomal drug for cattle. It was believed that it could kill kinetoplast DNA and prevent Trypanosoma infection in cattle. However, this truth has yet to be uncovered.
In molecular genetics, it is widely used in agarose gel electrophoresis experiments and karyotyping. Experiments such as PCR, DNA sequencing, restriction digestion and mutagenesis studies highly rely on the use of monitoring dye- EtBr.
Why is EtBr used in gel electrophoresis?
The intercalation property of the ethidium bromide allows us to use it in electrophoresis.
Mechanism of intercalation:
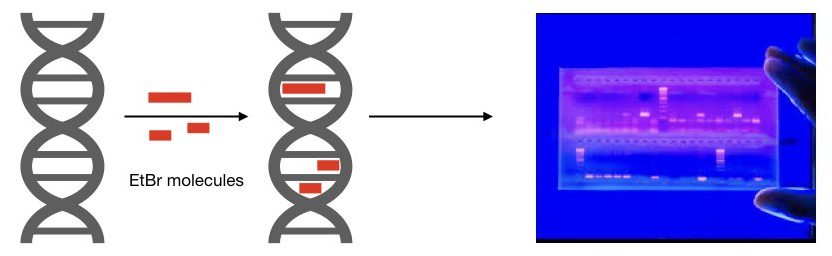
EtBr is a DNA-binding dye. It can potentially bind to a DNA major groove and between the stacked bases. EtBr has a tricyclic phenanthridine ring system. This ring interacts with the hydrophobic interior of the DNA bases with strong van der Waals bonds. Roughly one EtBr molecule will bind per 2.5 base pairs of DNA.
The hydrophobic environment present in between the base pairs increases the intensity of the fluorescence. Water is a highly efficient quencher of fluorescence. After intercalation between base pairs, under the hydrophobic environment, the EtBr removes water as it moves away from the buffer and emits fluorescence, increasingly.
It is also evident that it can only bind with the dsDNA (double-stranded) DNA and not with the single-stranded DNA. Thus, it is only used for the gel electrophoresis of genomic DNA, PCR products, restriction digestion, etc.
Notably, EtBr can affect mobility, molecular weight and charge of the DNA. Sigmon J and Larcom LL in 1966, had studied the effect of EtBr intercalation on double-stranded DNA. They used various concentrations of EtBr to check the mobility of DNA.
As the concentration of EtBr increases the mobility of DNA decreases. You can read their research paper here: The effect of ethidium bromide on the mobility of DNA fragments in agarose gel electrophoresis.
Their finding suggests that intercalated EtBr alters the conformational and physical properties of DNA. In spite of that, it is frequently used for DNA confirmation studies.
EtBr fluorescence:
EtBr emits orange fluorescence 25 fold and 21 fold higher for dsDNA and dsRNA, respectively. The fluorescence is detected at 205 nm wavelength. So the Fluorescence can be detected using the UV light.
Read more: What is Triple Helix DNA and How Does It Form?
Concentration:
With increasing the concentration of EtBr, the intensity of fluorescence increases. The standard concentration of EtBr for gel electrophoresis is 0.5μg/ml for 50 ml of gel.
Stock Concentration of EtBr
Ideally, a typical EtBr bottle comes in 10mg/ml concentration.
Working concentration of EtBr
We require a stock concentration of 0.5μg/ml for 50 ml gel. EtBr is a potential carcinogenic agent so we usually avoid preparing it, thus we do not prepare a working solution and instead use it directly.
If we are preparing 20 ml gel, for that we need 10 μg/20 ml gel. So what simply we do is, add 1 μL directly from the working solution in a gel.
Note that excessive use of EtBr can produce bad gel results. So it is important to use a good concentration that can produce a decent banding pattern.
Read more: 7 Purposes of Running Buffer in a Gel Electrophoresis + My Guide to Use it.
How to use EtBr in gel electrophoresis?
For two different types of gels (PAGE and agarose gel electrophoresis), two different techniques of adding EtBr are used. In gel electrophoresis, it can be directly added to the gel during gel preparation. In PAGE, the gel can be stained.
Read further: Clash of Gels- Agarose Gel vs PAGE Gel.
Direct gel addition:
- The required concentration of an agarose gel is melted in a flask.
- The solution is boiled and allowed to cool until it becomes touchable.
- 1μL of EtBr is added to the gel solution and mixed well. Don’t share the flask so vigorously to prevent bubble formation.
- Once EtBr is completely dissolved, pour the gel into a gel caster.
Staining procedure:
- In the staining method, the gel is separately prepared without adding the EtBr. After completion of a run, the gel is allowed to stain in an EtBr solution overnight.
- 0.5 μg/ml EtBr solution is prepared. The gel is submerged in the solution and allowed to stain at room temperature for 30 minutes or overnight (depending on the staining requirement).
- After that, the gel is destained in deionizing water for 30 minutes.
The staining method gives amazing results but is a tedious, time-consuming and costly method. Usually, we prefer to add EtBr directly in a gel. For PAGE and DNA sequencing, the staining method gives good results.
Storage and handling:
EtBr storage and handling is a crucial issue. It should be stored in adequate conditions and at the same time, should be handled with care.
Storage:
EtBr should be stored in dark conditions– away from the sunlight. Under sunlight, the Fluorescence activity is reduced due to the photobleaching effect. So the EtBr powder is stored at room temperature in a red or dark amber bottle. Whereas the solution is stored at 4ºC in a red or dark amber bottle.
In such adequate conditions, it remains stable for at least 5 years. However, it is important to monitor the activity and fluorescence intensity periodically.
Handling:
EtBr handling, as aforementioned, is a crucial issue in a lab. So every lab person should be trained for EtBr safety and handling procedures. Here are some of the important points for handing the EtBr.
- EtBr can be absorbed through the skin. Avoid direct skin contact with EtBr. To do so, wear gloves and a lab coat with long sleeves. Hand gloves should be of good quality.
- Wear eye protection while handling the EtBr. It can damage the eye.
- EtBr inhalation is also hazardous to health. Wear a mouth cap to avoid inhalation.
- If it accidentally drops on the skin or eye, immediately wash the area under running water.
- In addition, review or read the material safety and handling guidelines provided with the chemical.
Remember, EtBr can irritate skin, eyes, mouth and upper respiratory tract.
Limitations of EtBr:
Despite having amazing applications in molecular biology, EtBr has several serious limitations. The fluorescence activity can be reduced over time or under inadequate conditions. EtBr is mutagenic, carcinogenic, toxic and irritant.
EtBr Waste disposal:
Four types of EtBr waste are generated in a molecular genetic lab– EtBr solution, EtBr powder, used gloves and EtBr gel. For each type of waste, a different waste disposal guideline is followed.
It is important to minimize EtBr waste as it is a high health hazard. However, in case, if it is needed to dispose EtBr, follow the EHS (Environment, Health and Safety guidelines). Dispose of the EtBr in power by properly labeling it in a plastic container.
EtBr stained or containing gel can also be disposed using the same guideline.
Drain the EtBr solution with less than 1 μg/ml concentration in plenty of running water.
Collect the EtBr waste like the flask, containers, or gloves in a separate box and label it as a hazard. Discard it using the EHS guideline.
Charcoal filtration can be used to minimize the effect of liquid EtBr waste. <0.5mg/ml EtBr solution is passed down from the charcoal filter and collected in a separate container.
This solution is also labeled as a hazard and discarded using EHS protocol. Do not discard any EtBr-contaminated utility, chemical, or gel in a routine biohazard waste.
Wrapping up:
EtBr is an important chemical in a molecular genetic laboratory and plays a significantly important role. However, at the same time, it is hazardous too. By following the safety regulations, general lab safety guidelines and safety protocol, EtBr can be used without hesitation.
We in our lab and almost every molecular genetic lab use EtBr. So no need to worry about it. Just follow proper guidelines. I hope this article helped you learn to handle EtBr. Share this article and bookmark this page.
Sources:
Sigmon J, Larcom LL. The effect of ethidium bromide on mobility of DNA fragments in agarose gel electrophoresis. Electrophoresis. 1996 Oct;17(10):1524-7. doi: 10.1002/elps.1150171003.

