“DNA ligase seals two DNA fragments by creating a phosphodiester bond. Explore the concept of DNA ligase, its structure, function, mechanism and application.”
Replication is a process to copy DNA. In the previous article, we have explained the general concept of DNA replication. Now, we are moving ahead and discussing one crucial end player of the game– ligase.
After completion of all steps, starting from DNA unwinding to the synthesis, what do we need to do at the very end? We need to seal the gaps between newly synthesized DNA if occur. DNA polymerase is able to synthesize DNA but can’t seal the gaps.
As the replication isn’t a continuous process, at the end, gaps that occur on the lagging strand fragments should be filled. One enzyme, named Ligase, came into action very late and completes the process.
In this article, we will understand what DNA ligase is and how it works. I will also explain its role and working principle in replication. Lastly, we will discuss some crucial applications of ligase in genetic research.
Disclaimer: Information provided here is collected from peer-reviewed resources and re-presented in an understandable language. All the sources are enlisted at the end of the article.
Note: information on the T4 DNA Ligase has been removed from here and a better article is written separately. You can read it here: T4 DNA Ligase: Introduction, Functions, Applications and Protocol.
Key Topics:
What is DNA ligase?
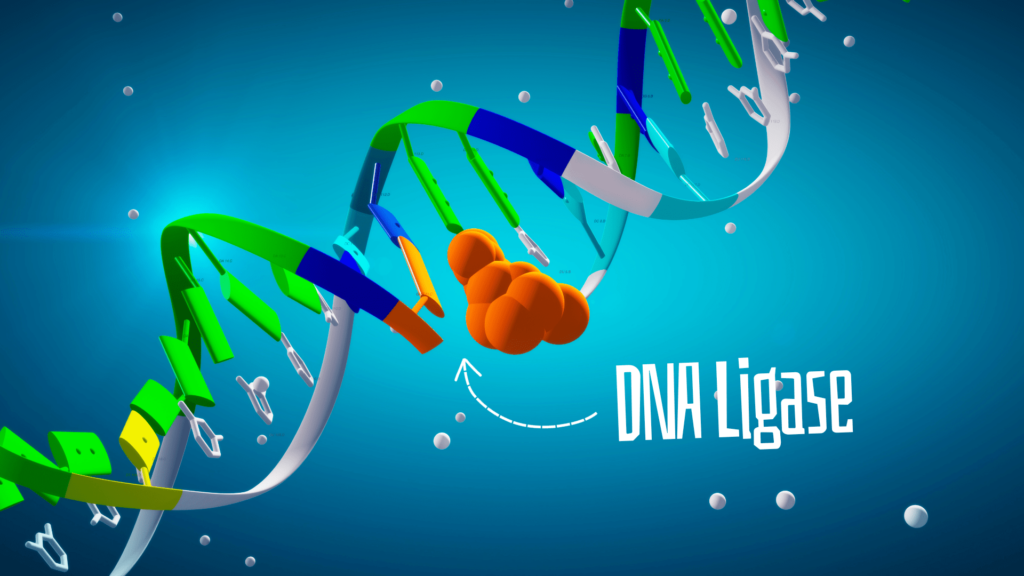
DNA ligase or just ‘Ligase’ is an enzyme of DNA metabolism that helps in ligating two DNA strands/fragments. Meaning, it seals the gap between two DNA fragments. Notedly, it can’t fill nucleotides.
It was discovered by Gellert, Lehman, Richardson and Hurwitz Laboratories in 1967. As replication is a universal process, the ligase is present in prokaryotes and eukaryotes. Eukaryotic ligase is an ATP-dependent enzyme.
In DNA metabolism, it is used in replication, recombination and DNA repair while in genetic research it is used in genetic engineering, cloning, mutagenesis and sequencing.
Read more: Replication 103: DNA Helicase- Structure, Function and Mechanism of DNA Unwinding.
Types of DNA ligase:
As aforesaid, it is present in eukaryotes and prokaryotes. Eukaryotic DNA ligases are DNA ligase I, II, III and IV while prokaryotic DNA ligases are LigA, LigD and bacteriophage DNA ligase.
Eukaryotic DNA Ligases:
| Ligase | Function |
| Ligase I | Is Involved in DNA replication preliminarily. It joins Okazaki fragments at the lagging strand and maintains DNA continuity. It’s present in the cell nucleus. |
| Ligase II | Is Involved in the DNA repair pathway. However, it doesn’t have its own gene and thus can’t be considered a true ligase. |
| Ligase III | Is Involved in DNA replication and repair pathways (Base Excision Repair). It’s a multifunctional DNA ligase. |
| Ligase IV | Is Involved in non-homologous end-joining DNA repair. It helps maintain genomic stability. It helps join double-stranded DNA breaks. |
Each ligase is encoded by various genes named ‘LIG’, however, note that as ligase II doesn’t have its own gene, it’s encoded by the LIG3 gene. Gene’s names and their locations are provided in the table below.
| Ligase | Gene | Gene Location |
| Ligase I | LIG1 | 19q13.3 |
| Ligase II | LIG3 | 17q12 |
| Ligase III | LIG3 | 17q12 |
| Ligase IV | LIG4 | 13q33.3 |
Prokaryotic DNA ligases:
Two well-known prokaryotic DNA ligases are LigA and LigD, and are present in Bacteria. Bacteria contain only a single LigA enzyme although some bacteria also contain LigD too.
| Ligase enzyme | Function |
| LigA | Helps in DNA replication and repair. |
| LigD | Helps in Double-strand break joining and non-homologous end-joining repair process. |
| The Bacteriophage Ligase | Phage often possesses its own ligase enzyme for replication and maintenance in the host. |
Structure of Ligase:
Let’s first discuss the general structure of eukaryotic DNA ligase and its various domains so that we can understand the roles of different domains during the ligation process.
In general, the eukaryotic ligase contains a catalytic or core domain, DNA binding domain, oligonucleotide binding domain, protein-protein interaction domain and Zinc finger domain (rarely).
The DNA binding domain is present on the N-terminal end of the enzyme. It helps to recognize the substrate or target DNA and identify the gap or nick. It contains positively charged amino acids which interact with the negative charge of the DNA.
This interaction facilitates correct DNA-enzyme binding.
The catalytic enzymatic domain plays crucial roles and has a major function in the entire process, thus known as a core domain too. It’s a central part of ligase and an active site where the ligation reaction occurs.
Thus, it governs the process of creating a phosphodiester bond between the 3’ OH and 5-P ends of DNA fragments. However, at initiation, it utilizes the ATP and converts ATP to release energy and pyrophosphate (PPi). This process is governed by the AMP-adding active site in the core domain.
Oligonucleotide binding site (OB) is often present in some ligases. Research suggests that it stabilizes the DNA-ligase complex and allows accurate ligation reaction.
Protein-protein interaction domain, present on the C-terminal end of the enzyme (if present) helps regulate ligase activity. It interacts with various proteins involved in replication or repair, thereby, regulating the overall enzyme activity.
Rarely present Zinc finger domain also ensures enzyme specificity by recognizing and binding with the substrate DNA. However, its significance is largely unknown.
Read more: Prokaryotic DNA Replication: Initiation, Elongation and Termination.
Mechanism of action:
Now, moving ahead, let’s understand how these domains collectively participate in the ligase-governed catalytic reaction.
First, the enzyme initiates the action by recognizing the substance DNA nick or gap.
The ligase DNA binding domain, thus, first came into action (and the Zinc finger domain, if present). The domain finds the gap or nick and stabilizes the enzyme for further action.
The 5’ end of the DNA fragment is known as ‘donor’ while the 3’ end is known as ‘recipient.’ Now ligase activates by the process of adenylation. By adding the AMP (adenosine monophosphate) to its lysine residue, it becomes adenylated.
Here it uses ATP as an energy source (or NAD+) for activation and preparation of an active AMP-enzyme complex. Phospho amino bond joins the AMP and enzyme’s lysine residue.
This whole process occurs in the enzyme’s catalytic or core domain.
Now, the active enzyme joins the donor (5’ OH) and the acceptor (3’ P) ends with a phosphodiester bond. The chemical reaction is shown in the figure below. Simultaneously, AMP (Adenosine Monophosphate) is released from the active site.
This is the exact process of nick or gap filling by ligase. It’s important to note that, although the ligase fills the gap by preparing the phosphodiester bond between two DNA fragments, it can’t add nucleotides.
Functions of Ligase
Largely, ligase governs the ligation reaction. It seals the broken end or gaps between two DNA fragments and henceforth, plays a pivotal function during replication, DNA repair and recombination.
Ligase in Replication:
Now, coming to the important part of this article. It has a large role in DNA Replication. We will not explain the process of replication comprehensively. I will just introduce major events that are important.
DNA synthesis at the leading strand occurs continuously. But DNA synthesis at the lagging strand occurs discontinuously. DNA polymerase synthesizes DNA into fragments known as Okazaki Fragments. These fragments are synthesized discontinuously, so gaps are created.
To fill those gaps, DNA ligase, after completion of lagging strand synthesis came into action. By following the chemical reaction, mentioned in the above section, ligase completes the reaction.
First, the N-terminus domain– DNA binding domain recognizes the gap in the DNA and identifies the substrate DNA. Then the core domain, which is also a catalytic domain, catalyzes the reaction and fills the gap between the 5’ and 3’ ends through a phosphodiester bond.
Check back on the mechanism and action part to understand the molecular mechanism behind ligation.
ATP conversion provides energy to the whole activation and catalytic process.
Ligase in DNA repair:
Ligase works effectively in both DNA repair pathways– base excision repair and non-homologous end joining. The mechanism of action remains similar as explained above.
Ligase in recombination:
Ligase also governs the recombination process in which genetic material is exchanged between two chromosomes. Here, in the final stage of the recombination, It seals the gap between exchanged chromatids.
Applications of Ligase:
- Enzyme DNA ligase is an important tool in genetic research, recombinant DNA studies and genetic engineering. Here I am enlisting several significant applications of Ligase.
- In genetic engineering, scientists prepare genetically modified organisms or products by introducing a gene into the target genome. Ligase seals the gap between the foreign DNA and the host genome effectively, thereby, succeeding genetic engineering experiments.
- It is also used in molecular cloning experiments to prepare recombinant plasmids, cosmids and artificial chromosomes.
- It is widely used in in vitro DNA repair studies in which various DNA damage events and repair pathways are tested.
- Ligase is an important tool for studies like site-directed mutagenesis in which a specific mutation has been introduced in a target DNA sequence.
- In techniques like sequencing or microarray, it’s used to prepare DNA or genomic DNA libraries. Known sequence adapters are ligated to the DNA fragments using ligase.
- In several tests like, Ligation-dependent amplification or Ligation Chain Reaction, the ligase is widely used.
Wrapping up:
Ligase is an important enzyme for DNA metabolism, especially for replication. Abnormal ligase activities abrupt the final DNA synthesis process and mess up the entire system.
Ligase is a significantly pivotal tool for genetic research as well. T4 DNA ligase is widely used in various genetic experiments. I have explained the T4 DNA ligase in this article’s previous version, but I have removed it and prepared a whole new article on the T4 DNA ligase topic. You can read that article, when available.
So that’s it for this article. I hope you like this article. Share it and bookmark the page. To strengthen your replication knowledge you can read previous articles of this series as well.
Next article: T4 DNA Ligase: Introduction, Functions, Applications and Protocol.
Sources:
Shuman S. DNA ligases: progress and prospects. J Biol Chem. 2009 Jun 26;284(26):17365-9. doi: 10.1074/jbc.R900017200. Epub 2009 Mar 27. PMID: 19329793; PMCID: PMC2719376.
Tomkinson AE, Sallmyr A. Structure and function of the DNA ligases encoded by the mammalian LIG3 gene. Gene. 2013 Dec 1;531(2):150-7. doi: 10.1016/j.gene.2013.08.061. Epub 2013 Sep 5. PMID: 24013086; PMCID: PMC3881560.