“The gene therapy is a method used to treat genetic disorders by introducing or removing a gene using gene transfer vectors.
Gene therapy is one of the challenging ongoing research in genetics, yet it is most promising.
The gene therapy method is used to treat genetic disorders. Scientists are using it to treat inherited genetic disorders like sickle cell anemia and thalassemia.
Various different monogenic and polygenic disorders are evolving every day which is why a successful gene targeting technology- gene therapy, is a must-needed technology in forthcoming years for mankind.
In gene therapy, the normal functional protein can be revived by replacing a mutant gene with a wild type one. However, it is not so easy as we are discussing, We have to face numerous challenges to do so. In this present article, We are going to discuss gene therapy and its types.
Also, various approaches to gene therapy are described in this article. Experiment on the model organism demonstrated that the use of successful gene therapy for hereditary disorders are still a long way off. However, promising results are gained for cancer and some monogenic disorders.
In 1989, the first patient was recovered through the gene transfer technique. In the present article, our major focus will be on different types of vectors used in gene therapy in addition to some of the examples of it.
Key Topics:
Key topics:
- History of Gene therapy
- What is gene therapy?
- Type of gene therapy
- The different technique of gene therapy
- Vectors for gene therapy
- Viral vectors
- Non-viral vector
- Stepwise process of gene therapy
- Application of gene therapy
- Limitations of gene therapy
- Challenges and Future aspects
- Conclusion
Commonly used abbreviations:
| Abbreviation | Full name |
| GOI | Gene of Interest |
| NIH | National Institute of Health |
| FDA | Food and Drug Administration |
| DMD | Duchenne Muscular Dystrophy |
| ITR | Inverted Terminal Repeats |
| CF | Cystic Fibrosis |
| AAV | Adeno-associated Virus |
| HIV | Human Immuno Virus |
| SB | Sleeping Beauty Transposon System |
| siRNA | Smaller Interfering RNA |
| HSV | Herpes Simplex Virus |
| CE | Control elements |
Note: The present article mainly focused on the technical aspects of gene therapy, if you want some basic idea and simple explanation, please first read our previous article:
A beginner’s guide to gene therapy: What is Gene Therapy? and How Does it Work?
Now let’s get into the topic,
History:
The concept of gene therapy was originated in the 60s by Rogers and Pfuderer. In 1961, K Lorraine had successfully introduced a functional gene in mammalian cells.
After several years of it, in 1971, Carl R. Merril experimented on Human Fibroblast cells and concluded that DNA could be inserted into the human genome for fixing the mutant gene.
Later on, in 1972 Theodore Friedman and coworkers successfully corrected the Lesch-Nyhan single gene disorder using gene therapy trials at the National Institute of Health.
The idea of gene transfer in a human was given by Martine Cline, who was one of the pioneer scientists in gene therapy research. He had also attempted DNA modification techniques in 1980.
In the year 1984, the first retroviral vector system was introduced by Richard Mulligan for delivering the foreign gene.
In 1990, a clinical trial of the world’s first approved gene therapy was performed under the close monitoring of NIH.
In the early ’90s, gene therapy gossip becomes stronger as it entered cancer treatment.
In 1992, Trojan and co-workers introduced the concept of using gene therapy in cancer treatment. They had postulated that the introduction of the transgene could trigger off the oncogenic activity.
In 2002, scientists had successfully treated adenosine deaminase deficiency through gene therapy. In the same year, sickle cell anemia mice were treated by introducing the artificial therapeutics gene.
The era for using a non-viral vector in gene therapy was begun when polyethylene glycol was first time used as a vector for the delivery gene into brain cells in 2003.
Do you know?
China was the pioneer in gene therapy, approved the first human gene therapy.
The summarised history of the gene therapy is given below,
2006: Two patients were treated for X-linked myeloid cell defect using gene therapy.
2006: Lentivirus is used for the treatment of HIV.
2007: Gene therapy trial has begun for inherited retinal disease.
2010: beta-thalassemia major child was successfully treated with gene therapy.
2012: FDA-approved gene therapy for the beta-thalassemia major treatment.
2014: Adeno-associated virus-mediated gene therapy is used for the treatment of choroideremia disease, a rare form of the x-linked recessive disorder of vision loss. Vision improvement in the patient was noticed after one year.
2014: Clinical trials for the treatment of sickle cell anemia were started.
2015: The gene therapy against beta-thalassemia (LentiGlobin305) is approved by the FDA. The therapy is mediated by the lentivirus.
2016: Adenosine deaminase deficiency was treated using the “strimvelis” gene therapy product which is later approved for the clinical trial by the European medical agency.
2017: Another successful clinical trial for sickle cell gene therapy was done by French scientists.
2017: FDA approved another gene therapy for ALL (acute lymphoblastic leukemia) named “Tisagenlecleucel.”The treatment was developed by Carl H. June.
2017: FDA approved another gene therapy named “Luxturna” for the treatment of Leber’s congenital amaurosis. It is the first in vivo therapy approved by FDA mediated by the AAV2.
2018: The World’s first-ever clinical trial of CRISPR-CAS9 was launched.
2019: The first gene-editing therapy in humans using ZFN was announced by the Sangamo Therapeutics scientist.
2019: Another gene therapy for the treatment of spinal muscular atrophy was approved by the FDA.
Commonly used terminologies:
| Terminology | Definition |
| Transgene | The exogenous gene is introduced in the target cell. |
| Vector | Vehicle or an agent to insert transgene in the defective cell. |
| Virion | Mature viral particles |
| Provirus | Reverse transcribed DNA of the retrovirus |
| In vivo | The process is performed directly on the living cell or organism. |
| Ex vivo | The process is performed on the tissue or cells outside the body. |
| Tropism | A biological phenomenon for the growth of an organism. |
What is gene therapy?
Introducing a new gene by replacing the mutant gene, is the prime goal of any gene therapy. In the present section, we will understand the mechanism of gene therapy and its types:
Definition:
“A process to recover the normal function of a gene by either transferring or removing a gene is known as gene therapy.”
Types of gene therapy:
Four types of gene therapy are most popular:
- Somatic cell gene therapy
- Germline gene therapy
- In vivo gene therapy
- Ex vivo gene therapy
The somatic cell and germline gene therapy are categorized based on the cell type involved in it while the In vivo and ex vivo gene therapies are categorized based on the method of application.
Somatic cell gene therapy:
The cells other than germline cells are somatic cells. Bones, blood, skin, internal organs, and other vital tissues develop from somatic cells that follow mitosis cell division. 46 numbers of chromosomes viz 23 pairs are present in somatic cells.
“In the somatic cell gene therapy, a mutant gene within somatic cells is replaced by a transgene.”
The change that we made through gene therapy remains lifetime only, it will not transmit to the next generation. And this is the limitation of the present therapy.
The somatic cells are not involved in reproduction, hence they can’t be inherited. Various ethical issues are involved in somatic cell gene therapy, though, the success rate is noticeable. Virus-mediated gene transfer and liposome-mediated gene transfer are widely used in SCGT (somatic cell gene therapy).
Although it can be applicable for all the somatic cell types, the common target is the bone marrow cells. Cells present in the bone marrow are the only cells that can be divided throughout life and produce different types of blood cells. This is the reason, SCGT success rate is more for blood born disorders such as thalassemia, sickle cell anemia, and hemophilia.
In the process, first, virus-like AAV (Adeno-associated virus)- commonly used as a vector in somatic gene therapy, infects the isolated bone marrow cells. Once the gene inserts at the target location, transformed cells are grown in a lab. The cells are injected back inside the bone marrow.
The broad overview of the entire mechanism of somatic cell gene therapy is shown in the figure below,
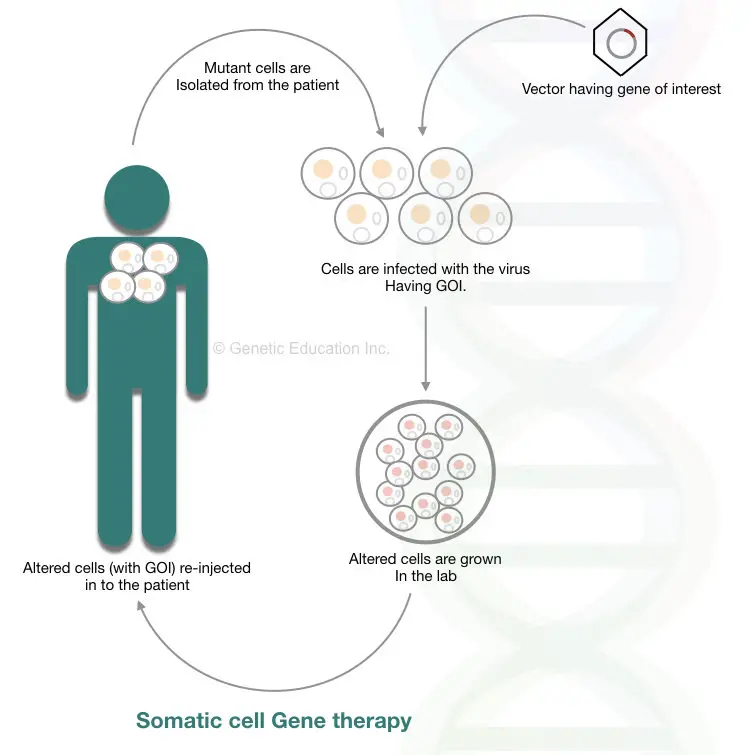
Somatic cell gene therapy has several limitations:
- a virus can combine with the host genome and infect the cell (Viral escape).
- Also, if a gene does not insert at the specific location, it may disrupt the function of the normal gene too.
- The chances of activation of oncogene and proto-oncogene are very high.
Do you know?
AVV can only alter the dividing cells as it directly integrates DNA into the nuclear chromosome
Germline gene therapy:
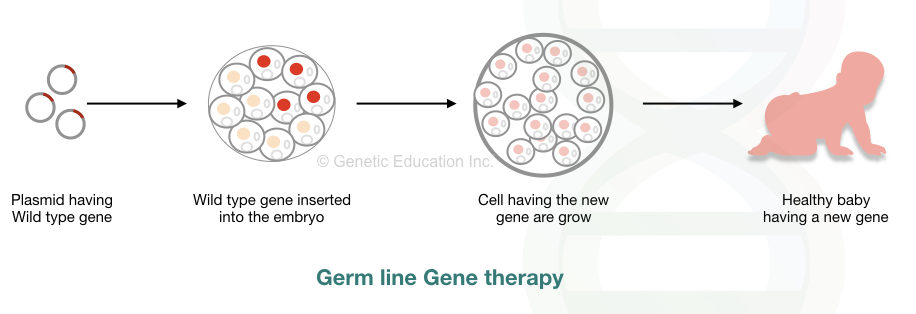
“Before splitting the embryo, the GOI can be introduced in germ cells (sperm or egg cell), fertilized egg cell or embryo, this method of transferring gene is referred to as a germline gene therapy.”
Consequently, the change made in the DNA of these cells can pass it to the next generations. As we are playing with live embryos here, some scientists believe that it is unethical. Germline gene therapy is prohibited worldwide.
However, the comprehensive overview of the method is like this:
Using viral vectors, cells of the reproductive tissues or un-split embryos are infected. The modified cells grow in the sterile lab environment, care must be taken while doing this. The modified cell must not be infected with other unmodified viral cells.
The modified germ cells are used for in vitro fertilization and other artificial assistive reproductive techniques. However, direct injection of a transgene into the embryo is preferred more. The mechanism of germline gene therapy is shown in the figure below,
In vivo gene therapy:
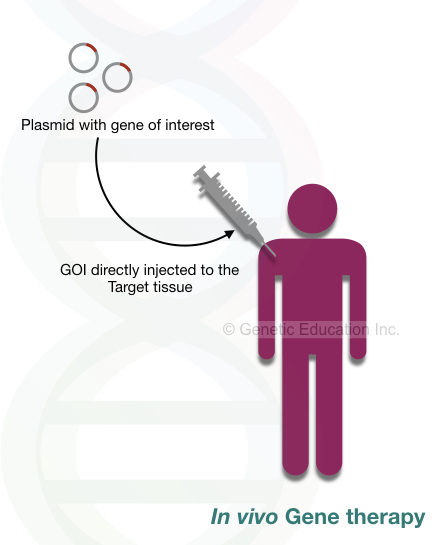
In in vivo gene therapy, the exogenous gene is directly inserted into the target cell. The gene of interest is introduced in our body through aerosols or injection.
The effect of in vivo gene therapy is restricted to some areas. It introduced GOI only to the affected area, not all the bodily tissues. The ideal examples of in vivo gene therapy are cystic fibrosis and Duchenne muscular dystrophy.
In cystic fibrosis, the exogenous gene (or transgene) is introduced through nasal spray (aerosol) whereas in DMD the GOI, a dystrophin gene is inserted into the muscle cell through the injection. Nonetheless, some surrounding cells are infected without any reason and cause some adverse effects.
Conclusively, in vivo gene therapy is not a good option for treating inherited diseases for now.
Ex vivo gene therapy:
In ex vivo gene therapy, affected cells are collected, modified in a lab (outside body system), and inserted back into our body. Here none of the gene therapy processes/steps is performed inside the body hence it is called ex vivo. A researcher can control the whole process, therefore the present method is safer and gives more control to scientists.
Although the technique is relevant for those cells which have the potency to divide. Hence only several types of tissues or cells can be altered using ex vivo. Bone marrow or blood cells are such types of cells often used for ex vivo gene therapy because the bone marrow cells can divide throughout life.
Why cell must be actively dividing?
The reason behind choosing actively dividing cells is that the transgene can spread faster in other cells. Therefore blood cells and bone marrow cells are the only choices for it. Because of this reason the ex vivo gene therapy is restricted to some of the blood-related disorders only.
Thalassemia, sickle cell anemia, thrombosis, and hemophilia can be treated using this technique. The comprehensive overview of the present method is shown below,
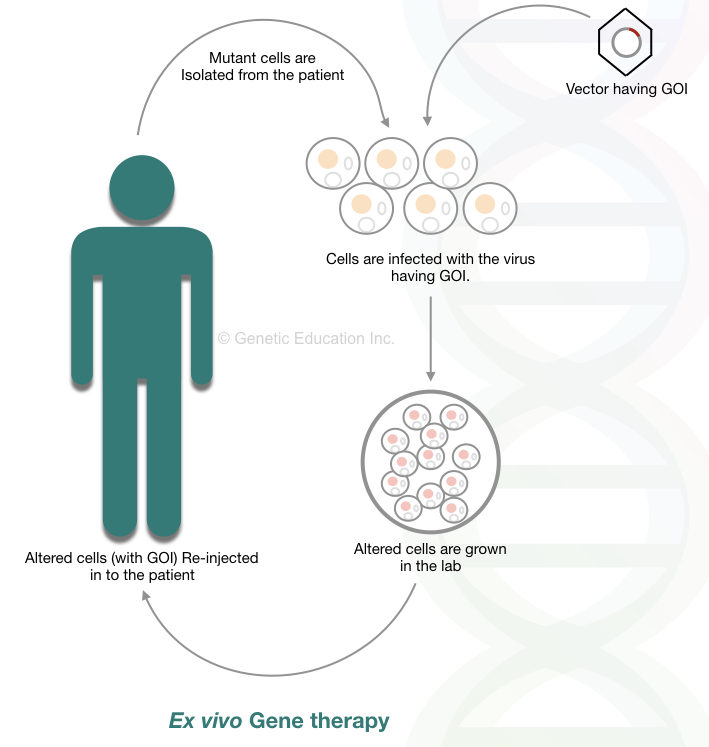
The steps of the ex vivo gene therapy are listed below,
The defective or mutant cells are isolated from the patient. Exogenous gene is inserted in the defective cell lines using viral vectors. Under strict sterile condition, altered cells are grown.
The transformed cells are selected and grow in such conditions where it isn’t infected with other viruses. The modified cells are now injected back to the patient’s body. The frequently used vectors for ex vivo gene therapy are retrovirus and AAV.
To use retrovirus, the ψ sequences of it are first removed, so that the virus can’t insert their own DNA into the host genome (We had discussed the whole mechanism in the next section of this article).
AAV is an efficient vehicle for ex vivo gene transfer because it can efficiently infect dividing cells. Cystic fibrosis and Duchenne muscular dystrophy can not be treated using it. The success rate of the ex vivo gene therapy depends on the rate of incorporation and expression of a transgene.
We will discuss hemophilia gene therapy in another article of this series.
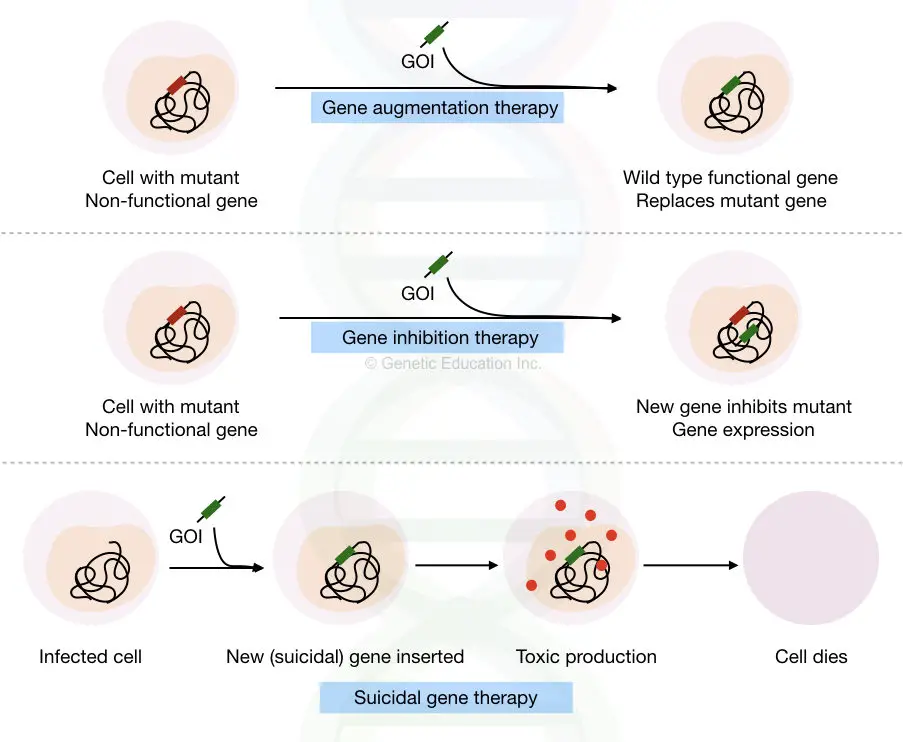
Different techniques of gene therapy:
Loss of function mutations and abnormal gene expression are two key reasons which cause genetic disorders. Based on the technique used, three other types of gene therapy are:
- Gene augmentation therapy
- Gene inhibition therapy
- Suicidal gene therapy
Gene augmentation therapy:
The natural function of a gene is lost due to some mutations known as a loss of function mutations, consequently, normal functional protein can’t be translated.
In gene augmentation therapy, the mutant (LOF gene) is replaced by the fully functional wild-type gene that translated a wild-type protein again. The present technique is majorly used for monogenic disorders.
Cystic fibrosis-like disorders can be treated using gene augmentation therapy.
Gene inhibition therapy:
Overexpression of a gene causes some life-threatening disease conditions like cancer.
Change in the methylation pattern or epigenetic profile of a particular gene results in the overexpression/underexpression of that particular gene.
In gene inhibition therapy, the overexpressed gene is inhibited by using,
- another gene or DNA sequence
- or by interfering with the activity of the product of that gene.
The present gene therapy is the best choice to treat inherited disease, infectious disease, and cancer. The overexpression of oncogenes can be lessened by using this method.
Related article: What is methylation?
Suicidal gene therapy:
In some types of diseases, it is very necessary to kill the cell, especially in cancer.
For those types of cells, some of the genes called suicidal genes are introduced to kill the cells.
The Gene produces a toxic product or protein that induces a strong immune response against that cell that leads to cell death. Suicidal gene therapy is specially designed to cure cancer. A brief overview of these three types of gene therapy is explained here,
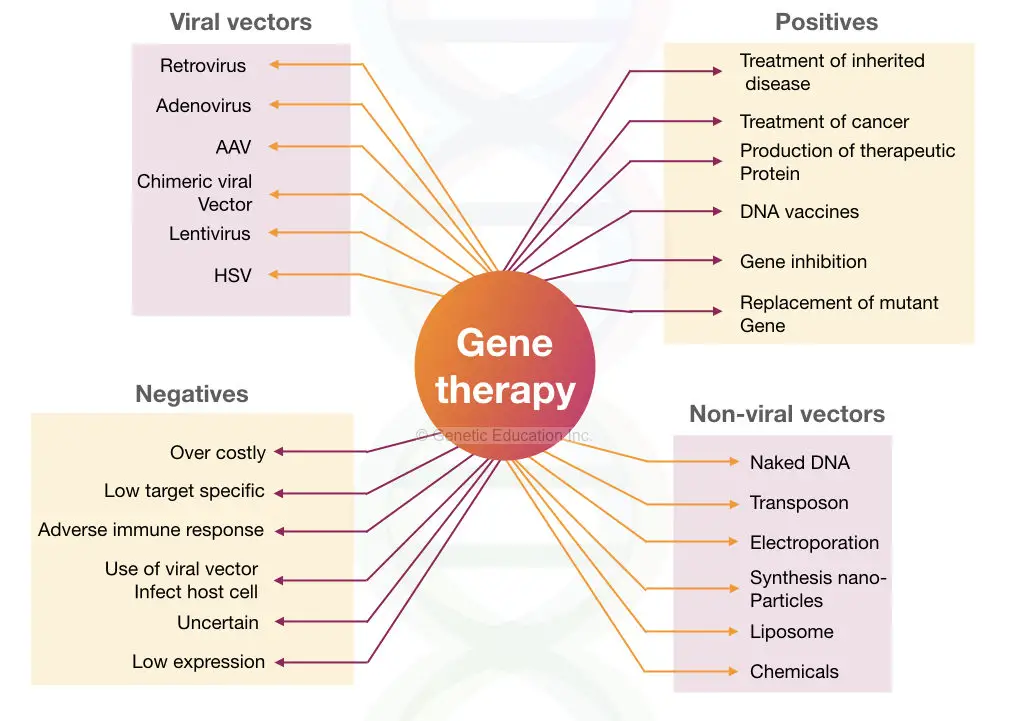
Gene therapy vectors (Viral and non-viral)
Viruses, liposomes, and naked DNA are some of the vehicles used to introduce transgene into the host genome.
The vehicles used to introduce the transgene is known as vectors, various criteria to select vector for gene therapy are discussed here. The utility of the vector depends on the factor enlisted below,
- The size of the exogenous gene (transgene)
- The efficiency of the delivery
- It will induce the host immune response or not
- The stability and longevity of the transgene
- Level of expression of a transgene
“The transgene which is selected for the gene therapy should not induce an immune response in the host, should have the capacity to carry larger transgene, and must have a high expression rate. “
The classification of the gene therapy vectors are given in the figure below,
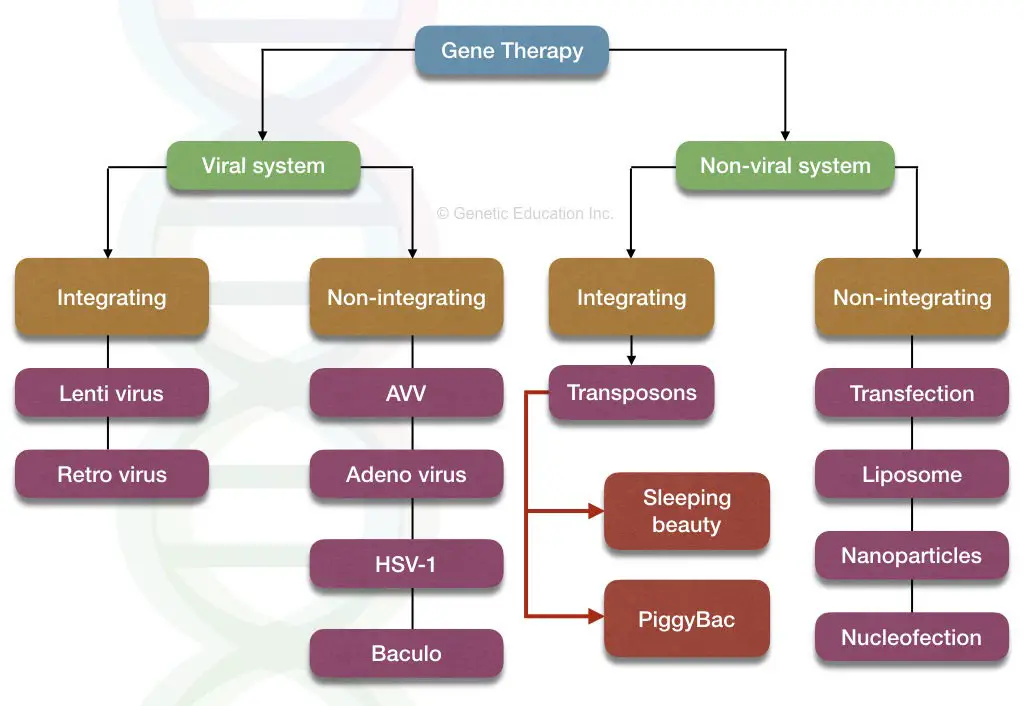
The vectors are divided into two broader categories:
- Viral vectors
- Non-viral vectors
Viral vectors:
Some of the common types of virus used in gene therapy are listed here,
- Retrovirus
- Lentivirus
- Adenovirus
- Adeno associated virus
Now, let’s discuss each vector in detail,
1. Retrovirus:
The retrovirus is an RNA virus, the genome of it is made up of RNA (not DNA). It has two RNA molecules in its genome. Therefore it is also also known as retrovirus-mediated gene therapy.
By the mechanism of reverse transcription, the RNA forms DNA (more specifically called cDNA), and the DNA is integrated into the genome of an organism.
Structurally, the retrovirus contains gag, pol, env genes, long terminal repeats (LTRs) on both the side and Ψ sequences.
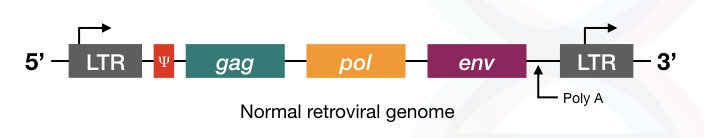
The Pol gene encodes for the reverse transcriptase enzyme.
The gag gene encodes for the viral core protein
The env gene encodes for the envelope protein present on the surface of the virus which helps in the recognition of the receptor present on the target cell.
The Ψ sequence is required for the packaging of viral particles, therefore these sequences are very important for the virus to infect the host cell, although these sequences are non-coding.
The LTR sequences present on both ends help reverse-transcribed DNA to integrate into the host genome.
About “retro”
The structure of retrovirus and retrotransposons are almost similar, indicating that retrotransposons are originated from the retrovirus.
Read more on retrotransposon:
The structure of retrovirus is given in the figure below,
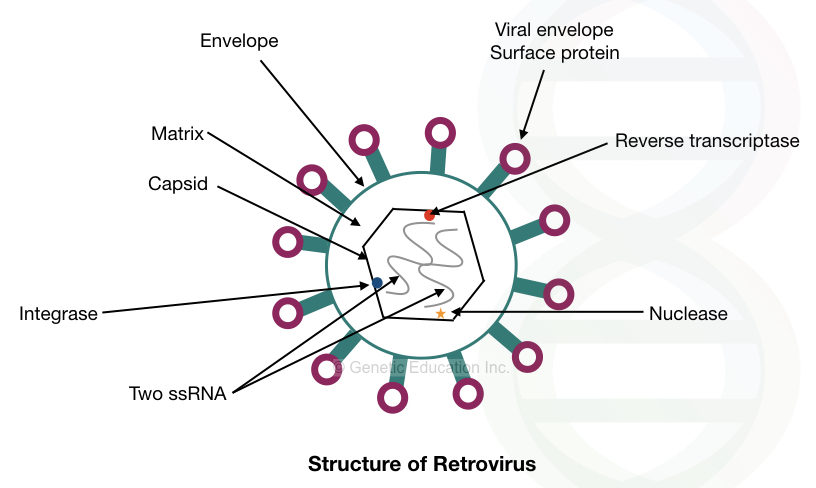
The retrovirus is one of the best choices for gene therapy, as it can integrate DNA efficiently into the host genome.
The unpacked naked retrovirus is used to fulfill the purpose. If the packaging assembly viz the Ψ sequences are present, it actively involves in the viral-packaging and the virus creates a new viral particle inside the host cell.
Therefore this must be removed first.
Secondly, the virus can activate the oncogenes present in the genome if it is integrated upstream to it.
So once the cell is infected, the altered cell lines must not be contaminated with the replication-competent virus.
Note: The replication-competent virus has the ability to replicate in the host cell.
For the gene therapy experiments three different types of plasmids are used.
- One having the 5′ LTR and an env gene, without the Ψ sequences (plasmid 1).
- Second with the 5′ LTR, gag, and pol gene, without the Ψ sequences (plasmid 2).
- Third with both 3′ and 5′ LTR, promoter sequence, a gene of interest, and the Ψ sequence sandwiched between both the LTR region (plasmid 3).
See the figure below,
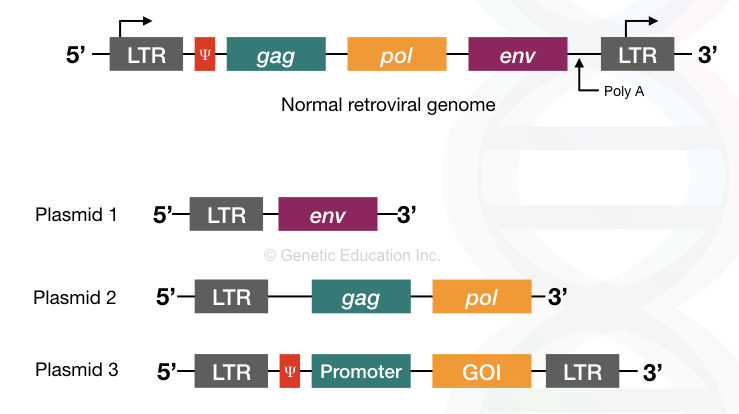
Here the first and second plasmid does not have the Ψ sequence, therefore, it can not produce virion. However, the essential pol and gal gene provides for replication.
The last plasmid contains a gene of interest with the promoter and the Ψ sequence so that the mature virion particles are formed but with the GOI (not gag, pol, and env).
After inserting into the host cell, the pol gene encodes for the reverse transcriptase and ensured the integration of target DNA into the host genome.
Interestingly, you have noticed one thing that here in both non-virion plasmids only 3′ LTR is present whereas in the third type of plasmid both LTR are present.
The LTR sequence is necessary for the insertion of DNA into the host genome. Additionally, the promoter for the retrovirus is located on the 5′ LTR region necessary for the retroviral gene transcription. Therefore for inhibiting such a viral activity, only 3′ LTR sequences are used in both types of plasmids.
Here the chance of contamination is very high hence each cell line (altered one) is cross-checked for the presence of replication-competent virus before being injected into the patient.
This method is the most advanced and safer.
In another method, along with the retrovirus, the helper virus is used. The env gene was removed from the retrovirus and used to infect the target cell.
The helper virus is also inserted along with it, use as a vector to insert the gene of interest. In this method, the chance of infection is very high as the cell line can be contaminated with both retroviruses as well as a helper virus.
Though retrovirus-mediated gene therapy is more promising, it has several shortcomings.
It can only infect the dividing cells because it inserts DNA direct on the chromosome. And therefore it is not a good option for DMD and CF, we had already discussed it in the ex vivo gene therapy.
It can only insert DNA up to ~8kb, therefore the size of DNA transfer is limited.
The retrovirus infects all types of body cells, consequently, it is not accurately target-specific. Sometimes the GOI does not reach the target cell, sometimes it landed in some other type of cell.
Besides, the integration is random, it integrates DNA at any random site in the genome. hence, if it is inserted near the cellular oncogene, it might activate it and can cause cancer.
Also, the retrovirus itself can infect the host cell if it is contaminated.
The target specificity can be achieved by modifying the viral envelope protein. Murine leukemia virus is ordinarily used as retrovirus in gene therapy.
Read the related articles:
2. Adenovirus:
Apart from the retrovirus, yet another good choice vector for gene therapy is the Adenovirus.
The genome of Adenovirus is made up of double-stranded DNA. The size of the genome is 35kb, which is surrounded by the icosahedral capsid made up of 12 different proteins.
It is beneficial over the retrovirus because it can naturally infect the non-dividing cells, especially, cells of respiratory and gastrointestinal tracks. This is the reason, the present vector is best suited for cystic fibrosis. One other benefit the adenovirus provides among all other vectors is that it can evade the host immune response.
It is also highly target-specific and has controlled integration which means it can only infect or integrate the DNA into its host cells, not other surrounding cells.
Among 50 different serotypes, serotypes 3 and 5 have a higher degree of tropism for the respiratory tract cells.
However, due to its harmful effects, it is very necessary to disable the viral replicating mechanism. The viral gene expression can be divided into early and late phases, for understanding this we have to understand its structure.
The virus has the 100bp terminal inverted repeats between which the 35 kb DNA is present.
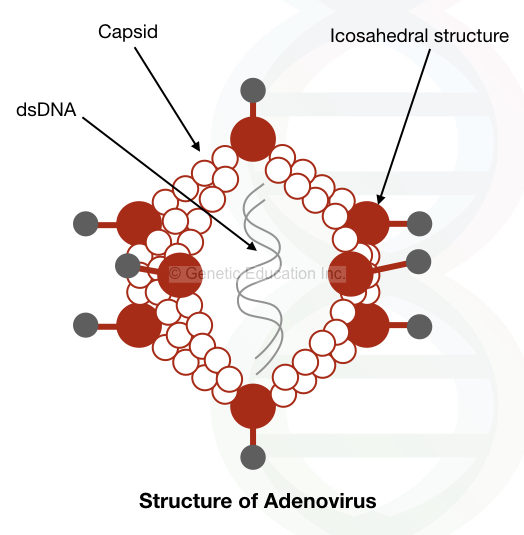
The expression of the adenovirus is divided into two phases the early infection phase and the late phase. The early gene expression is too low and derived from the E1, E2, E3, and E4 regions of the genome.
The rest of the region is involved in the late expression of the virus.
Clinical trial on the adenovirus revealed that even in the inactive stage adenovirus prompts a strong immune response.
3. Adeno-associated virus:
The genome of the Adeno-associated virus is made up of the single-stranded DNA contains only two genes “rep” and “cap”.
Also, the terminal repeats are present on both ends of it.
The rep gene encodes for the protein that helps the AAV to integrate into the host genome, especially on chromosome 9.
The cap gene encodes for the protein that constructs the capsid of it.
Interestingly, the AAV is naturally non-replicative, it required one helper virus to perform its function. The adenovirus is used to do this function along with the AAV.
Since the AAV can infect both dividing and non-dividing cells, it is the best alternative to the adenovirus. Majorly, it infects the cells of upper respiratory airways with a long-lasting expression of more than 6 months.
For gene therapy, the rap and cap gene of the AAV is replaced by the transgene. By removing the rep gene, the virus loses its specific integration power on chromosome 9.
The genomic structure of AAV is shown in the figure below,
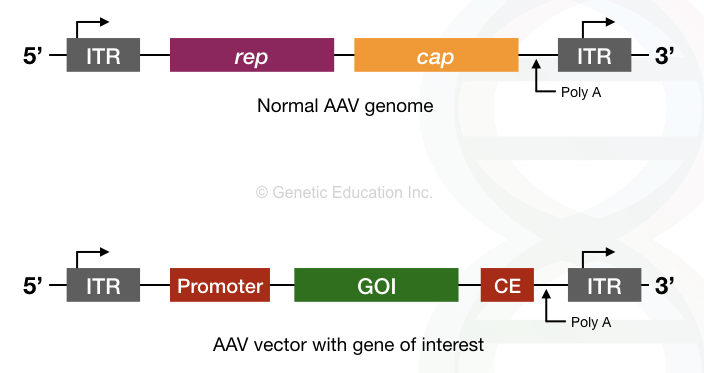
Due to the long-lasting expression of AAV, it is now allowed in human trials for hemophilia A gene therapy.
4. Lentivirus:
The lentivirus is another form of retrovirus that can even infect the non-dividing cells. HIV is one of the best examples of Lenti-retrovirus.
RNA is the genetic composition of a lentivirus that carries env, gag, and pol genes. The Lentivirus only infects the T cells.
Practically, using HIV for gene therapy is not a good choice.
A summary of the viral vectors:
| vector | DNA carrying capacity | Positives | Negatives |
| Adenovirus | 8kb to 35kb | -Infect both dividings as well as non-dividing cells.
-Chance of infection is less | -Provoke strong immune response.
-Transient expression |
| AAV | Up to 4.5kb | -Longer transgene expression.
-Non-pathogenic -Broad tropism | -Smaller DNA carrying capacity.
-Required a helper virus |
| Retrovirus | Less than 8kb | -Stable integration
-Infect replication cells -Suitable for ex vivo treatment | -Oncogenic activity
-Uncontrolled integration -Can not infect non-dividing cells -Adverse effect -Provoke immune response |
| Lentivirus | 8kb | -Infect proliferating, non-proliferating and bone marrow cells.
-Self inactive | -The DNA carrying capacity is less.
-The chance of infection is high. -Provoke immune response. |
| HSV | 30kb | -Safer
-Higher DNA carrying capacity -Suitable for in vivo gene therapy | -Difficult to produce |
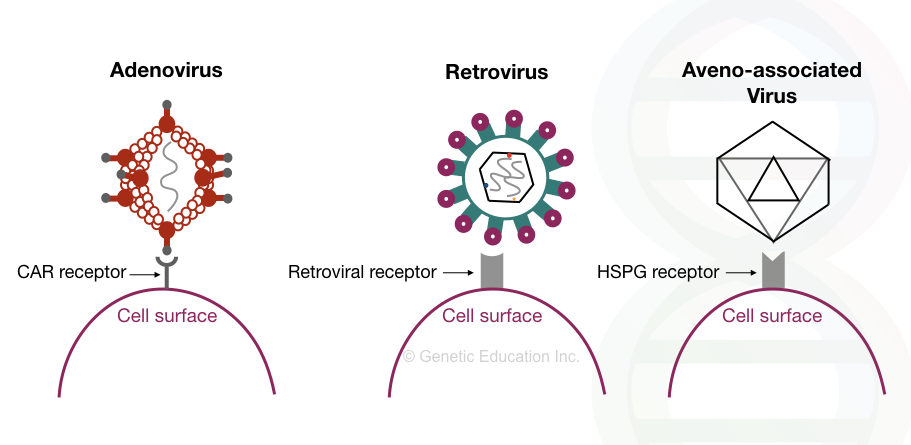
The image given below shows how the viral vectors insert the gene of interest into the host genome by interacting with the receptors present on the surface of the cell.
Non-viral vector:
Liposome, naked DNA, nucleofection, and transposons are some of the non-viral vector-mediated methods used for gene therapy. Why the non-viral vector will be one of the best opportunities for gene transfer?
The non-viral vectors are non-toxic, non-immunogenic, and tissue-specific. Also, it is easy to design and apply them. Some of the non-viral vectors are discussed here below,
Liposome:
The liposome also called lipoplex-mediated gene therapy is an artificial technique non-infected to the host cell. The liposomes are artificially synthesized molecules typically 0.025 to 2 μm in size, made up of lipids.
The DNA can not directly be inserted into the cell because of the negative charge present on a cell surface and DNA itself. Hence naturally both repel each other. The liposome is used to solve this problem.
The lipid molecule is hydrophilic as well as hydrophobic in nature which protects the interior aqueous phase. The head of it is hydrophilic while the tail is hydrophobic. The liquid solution of DNA is encapsulated with the liposome lipid bilayer which helps in penetrating it.
Nonetheless, due to its smaller size, the liposome isn’t a good choice for the larger size of gene transfer. Also, the liposome is non-attractive for the DNA as well as the cell surface. This problem is encountered by introducing a positive charge on the hydrophilic domain of the lipid.
A new type of upgraded version of the liposome has been designed and named “lipoplex”.
The lipoplex attracts both the DNA as well as the cell surface, consequently, a more stable complex of lipid-DNA is created.
This tube-like structure efficiently transfers DNA into the target cell. Plus, lipoplex is non-immunogenic because of this reason, it is the best choice over the liposome and virus-based vector.
Furthermore, it is easy to prepare and can transfer a large amount of DNA.
The major disadvantage of the lipoplex is that it is not as efficient as virus-based gene therapy.
Transposon:
Transposon-Mediated gene therapy is one of the emerging therapies after the CRISPR-CAS9.
Why transposon?
The transposons are mobile genetic elements that can move from one location to another into the genome. It also contains coding genes and terminal repeats like viruses. However, almost all transposons are inactive for a long.
Scientists had discovered active transposon from the fish fossil and named it as sleeping beauty transposon. The SB system is capable of inserting DNA into the host genome without any side effects.
It escapes the host immune system too and delivers a gene of interest efficiently at the target site. Although the method still has many loopholes and limitations that need to be perfected before any pre-clinical trial.
The general mechanism of the SB system is shown in the figure below,
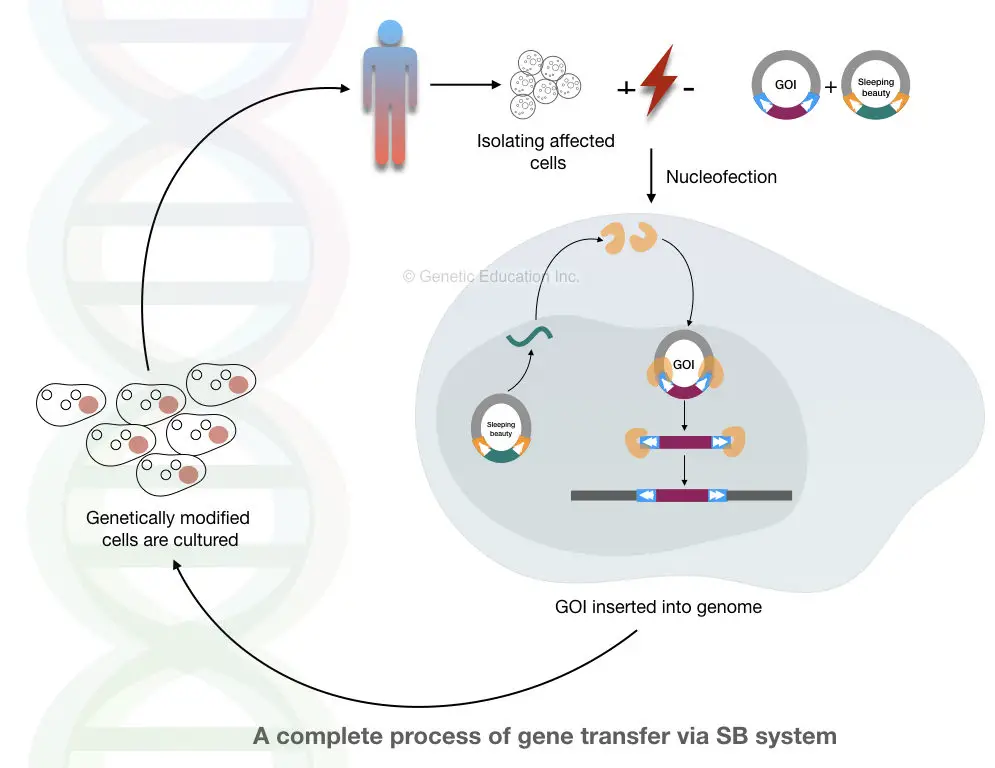
Nevertheless, systems like SB transposon and CRISPR-CAS9 will become more effective in the future.
We had explained the sleeping beauty transposon in our previous article, the article contains all the information on the SB system, starting from their discovery to its mechanism of action.
Naked DNA:
During the experiments on mice, scientists had observed that the naked DNA was directly injected into the mice muscle cells through the lesions present on the cell surface.
Although theoretically, it should not possible as both the DNA and the cell surface contain negatively charged molecules that repel each other. Other scientists believe that naked DNA will be useful for the production of therapeutic proteins.
The transgene can be inserted into the muscle cell for the production of proteins such as insulin and thrombotic factors. Nevertheless, enough research data are not available at present in favor of naked DNA mediated gene therapy.
Read our article on naked DNA: Naked DNA Mediated Gene Therapy.
Some of the other non-viral vectors mediated gene therapy methods are discussed here.
The non-viral vectors are further divided into three broader categories.
- Physical methods
- Chemical methods
- Synthetic molecules
1. Physical methods:
Several physical forces or procedures such as needles, gene guns, electroporation, ultra-sonication, and laser photo-poration can be used in gene transfer.
Electroporation:
The first attempt of in vitro electroporation was done in the year 1982 whereas the first attempt for the in vivo electroporation was demonstrated in the year 1991. However, the method is known since 1960. The basic method for both in vivo as well as in vitro electroporation is the same.
By applying the high electrical current for breaking the cell wall, pores are created on the cell surface in a second. The higher the pulse duration faster the pore forms. The duration of pulse and amplitude decides the permeability of the membrane for the gene transfer.
The transgene can be delivered either intramuscularly or intradermally. Sometimes intratumoral gene transfer can also be performed in the case of cancer. The electroporation readily delivered plasmid DNA into the cell. The field strength and the pulse vary from tissue type.
The in vivo electroporation method having a higher specificity and success rate directly injects the plasmid DNA into the target tissue.
Contrary, the method is restricted to some of the tissues, it is not accessible to the internal organs.
The in vitro electroporation is not as impressive as the in vivo therapy.
The method is also known as an electro-gene injection, electro-gene transfer, or electrical mediated gene therapy. We have written an amazing article on the present topics, read it here: Electroporation- A Modern Gene Transfer Technique.
Sonoporation:
The method is often known as sonication, was first described in the year 1954 for transdermal drug delivery. For the cellular DNA engulf, a temporary cell membrane permeability is created using ultrasonic waves.
After each round of sonication, the energy absorbed by the tissue results in the locally temporary heating that disrupts the cell membrane and produced holes. The process of sonoporation-mediated cavitation is called acoustic-mediated cavitation.
The ultrasound frequency for the gene transfer is 1-3 MHz with 0.5 to 2.5 W/cm2 intensity.
The use of artificial microbubbles made up of the lipid layer and gas-filled core makes the method more advanced and efficient.
The use of the surface stabilized such as synthetic lipid or polymers, phospholipid or albumin makes the microbubble more powerful for gene therapy. The transgene is introduced in the microbubbles by charge coupling, incorporating it into the shell or lumen.
The Microbubbles are 1 to 6 μm in diameter having white blood cell-like resemblance.
The success rate of the sonoporation depends on the:
- The intensity of the ultrasound
- Frequency of ultrasound
- Size of DNA to transfer
- The structure of the microbubble
- Duration of sonication
The method is highly site-specific, non-invasive, and safer. Even the method is applicable for the internal organs also without any surgical operations.
The method is readily available for the vast majority of tissue types such as muscles, heart, cornea, brain, and kidney tissues.
Furthermore, along with the microbubbles using tissue-specific receptors, antibodies and ligands increase the specificity of the method.
Gene Gun:
The gene gun method is first introduced in the year 1987.
The method is also known as ballistic DNA transfer, micro-projectile gene transfer or DNA-coated particle bombardment.
Highly pressurized gas and metal ions are two components of the particle bombardment gene gun method. Also, instead of highly pressurized gas, an electrical current or electrical discharge method can also be used.
The microparticles made up of silver, gold or tungsten deliver the transgene under the speed of highly pressurized gas (helium).
The Gene gun method is efficiently used for the intradermal, intramuscular or intratumor gene transfer.
The success rate of the present method depends on the gas pressure and velocity, size of the microparticle, size of the transgene, and the dose of injection.
1μm metal particles precisely transfer the transgene to the target. It is routinely used in ovarian cancer nowadays.
Photoporation:
In the photoporation method, a transgene introduction is permitted by the laser-induced pores in the cell membrane.
The success of the gene delivery depends on the frequency of laser light and the focal length.
Due to the lack of documented evidence and research data, the method is not used so often in gene therapy. Although, it is as effective as electroporation.
Hydroporation:
A large amount of DNA solution is directly injected via hydrodynamic pressure. The pressure creates a pore through which the DNA is inserted into the host cell. The method is commonly for gene transfer in the hepatic cells.
Needle:
The needle method is first used for naked DNA insertion thus it is more suitable for gene insertion in skin, muscle, liver and cardiac cells.
The gene of interest is injected directly through the needle without using any physical method. The method is simple yet effective.
However, the efficiency is too low as compared to other gene therapy vectors.
Magnetofection:
In this method, the magnetically charged particles copulated with the GOI. Under the higher magnetic field, the transgene is inserted into the cell or cell line.
The magnetoreception method is more suitable for ex vivo applications.
Some of the symbols used in this article:
2. Chemical method:
Here we are not discussing all the chemicals used in gene therapy. Gold particles, silver particles, silica and calcium phosphate are used as chemical agents for gene delivery.
These particles efficiently transfer the gene into the cytosol by complexing with it or by protecting it from nucleases or other enzymes.
3. synthetic nanoparticles:
Synthetic nanoparticles are another optional source for gene delivery that are safer and easy to use.
Cyclodextrins like cyclic natural nanomaterials can interact with the DNA having low immunogenicity. Therefore it is one of the best naturally available nanomaterial for gene therapy.
It is very essential for a foreign particle to escape from the endocytosis, the process that kills it.
Polyethyleneimine (PEI) is a gene delivery vector that helps in escaping GOI from the endosome. However, due to the presence of high positive charges on it, the vector is less effective.
Polyethyleneglycol (PEG) is one of the best nanoparticles used in gene therapy. It can efficiently transfer DNA to its target location.
It is majorly used in the delivery of siRNA.
Besides all these, Some of the peptides and proteins are also used for gene transfer.
Other nano-particles such as dendrimers, polymethacrylate, chitosan, and cationic synthetic lipids are used as gene delivery vehicles too.
Read more: PCR reaction: Ten secrets that nobody tells you
The process of gene therapy:
Any of the gene therapy experiments can be divided into 5 different steps:
- Selection of GOI
- Selection of plasmid
- Selection of vector
- Selection of method
- Selection of technique
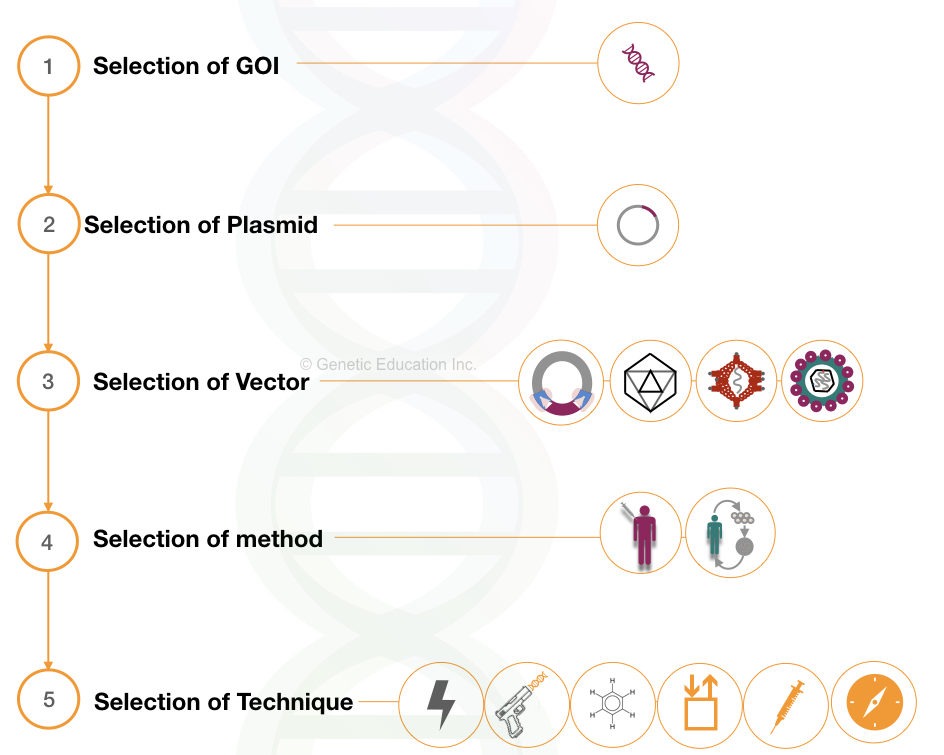
1. Selection of GOI:
One of the most crucial steps in the gene therapy experiment is selecting the gene of interest.
We have to select the appropriate GOI based on the disease type.
For instance, a single gene can be transferred efficiently rather than two or more genes. Therefore selecting monogenic disease yields more promising results.
Furthermore, the size of the gene also matters a lot to succeed in the experiment. The gene transfer efficiency of smaller fragments is very good as compared with larger DNA fragments.
The GOI must have the following characteristic
- The gene must have high AT-rich sequences.
- GC content must be less than 50%
- The gene must not contain a large number of exons
2. Selection of plasmid:
Plasmid plays an important role in delivering the gene of interest at its specific location into the host genome.
The plasmid selected for the experiment must have a higher transgene carrying capacity.
Also, it possesses some of the important sequences needed to insert the DNA.
It must have,
- Promoter sequences (specific to the gene of interest)
- ITR (inverted terminal repeats) which is needed for the recognition of the target site in the genome.
- An antibiotic resistance gene
- Control elements
- Genes essential for viral enzymes.
3. Selection of vector:
Vector selection is another big factor for gene therapy. The vector selection process is based on the type of gene we want to insert.
Although the viral vector gives an excellent delivery rate and integration efficiency. It is not so safe.
Furthermore, the selection of vectors is also based on the size of the GOI.
For instance, if the size of the transgene >35 kb, the adenovirus should not be recommended because its gene carrying capacity is lower than this.
Causion
Care must be taken while using the viral vectors especially the retrovirus as it has severe infectivity.
4. Selection of method:
After selecting both GOI as well as the vector, decide the method of the gene transfer.
If the target tissue is a somatic cell, use the somatic cell gene transfer method.
Choosing ex vivo or in vivo is depends on the transgene which you want to insert.
For example, if the transgene is for cystic fibrosis, in vivo gene therapy method works excellently, however, the same method does fail for DMD or AIDS.
Contrary to this, ex vivo gene therapy gives more precision for a disease like hemophilia.
The selection of the method is based on the:
- Type of disease
- Type of transgene
- Type of tissue
- Chance of infection
Generally, ex vivo gene therapy for AIDS does not recommend due to the high-risk factor associated with it.
5. Selection of technique:
The fifth step of the gene therapy experiment is the selection of techniques.
If the non-viral vector is selected as a method, you need one of the techniques from sonication, electroporation, magnetoception, gene gun or liposome.
The DNA can not directly be inserted into the host cell, so we need to create pores on the cell surface.
Electroporation is the best technique for all types of gene transfer experiments with non-viral vectors.
Even, it works more efficiently along with the liposome too.
Experimental setup:
The experimental setup is as crucial as the above-listed steps.
For the gene therapy experiments we need a highly contaminant-free, sterile, and sophisticated setup.
A state-of-the-art laboratory facilitating gene therapy must be equipped with all the utilities and safety setups.
Also, the experiments must be performed under the supervision of the experts.
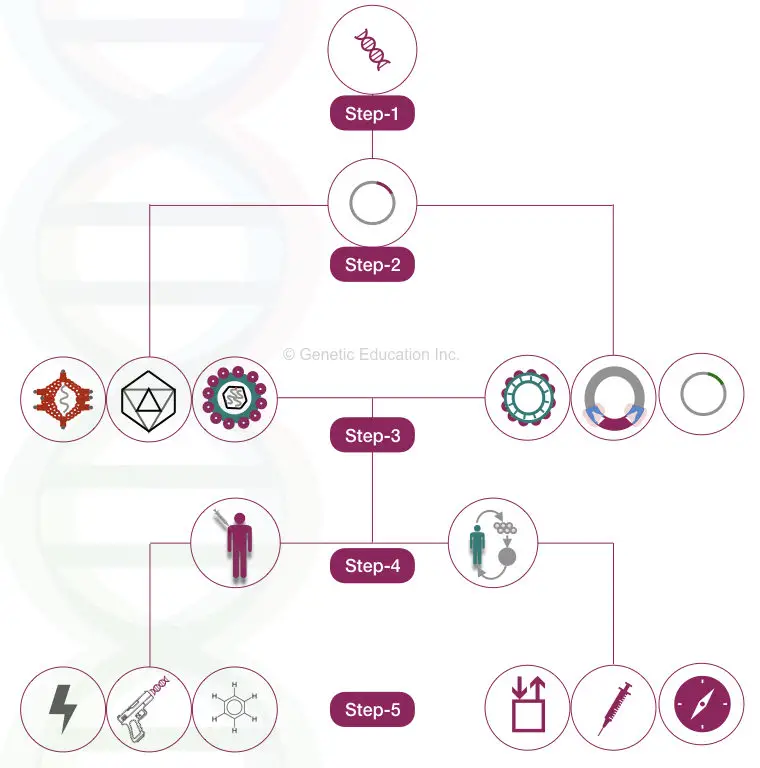
Steps of the gene therapy are shown in the figure below,
Possible applications of gene therapy:
The faulty or the mutated gene is replaced by the healthy gene using the gene therapy method. The method can be used for the diagnosis of disease if approved globally.
It is used in the diagnosis of inherited diseases such as cystic fibrosis, Duchenne muscular dystrophy, muscular atrophy and hemophilia. Furthermore, efforts are being made to develop gene therapy against monogenic disorders such as thalassemia and sickle cell anemia.
The in vivo gene therapy or viral vector-mediated gene therapy is a good option for treating diseases like Parkinson’s disease, Alzheimer’s disease, and brain tumors.
Nowadays gene therapy opened doors for dentistry. It is used to grow new teeth, bone repair and teeth regeneration.
DNA vaccines are another futuristic application of gene therapy, a naked DNA is directly injected for the production of therapeutic proteins such as insulin and thrombotic factors.
Treating cancer using transgene is another utilization of gene therapy in which the expression of an oncogene is suppressed by using a transgene.
Also, the infected cancerous cells are likewise killed by expressing a toxic producing gene into those cells. Consequently, it also promotes apoptosis of the infected cells.
In addition to this, cardiac disorders, neurological disorders, infectious disorders and autoimmune disorders can be treated using gene therapy methods.
Limitations of gene therapy:
Each type of gene therapy has different problems, therefore, fully functioning gene therapy is still not available for a clinical trial on humans.
The viral vectors used in gene therapy can infect the host cells and produce a strong immune response against them.
Furthermore, the transgene is not expressed all the time.
Due to the short-lived nature of transgene, the success rate of gene therapy is too low.
Gene transfer experiments are restricted to live human embryos because of the ethical issues associated with it.
Rapid integration of transgene is not possible, therefore, we don’t know when will the transgene be expressed.
Further, not all the cells of particular tissue accept the transgene.
Random integration to other locations can produce an adverse effect, gene activation/deactivation or oncogene activation.
The cost of gene therapy is very high, at approximately $ 100,000 per therapy (the insurance company doesn’t cover it).
Related articles:
Challenges & future aspect of gene therapy:
Undoubtedly, gene therapy is an effective way to prevent any disorder but with it, so many challenges are involved.
Safety is one of the first challenges associated with it. The viral vector-based gene transfer is more effective but can infect the host cell or stimulate an immune response.
Therefore it is very important to design a safer vector.
Since non-targeted insertion can cause serious health problems, the GOI must be incorporated at a specific location.
It is also a challenging task to insert the gene at a specific location.
The gene must be inserted at a particular location, just switch on the normal function of it and do not interrupt other gene’s function.
The new gene can perhaps raise the oncogene expression which results in carcinoma. Therefore, the insert should not be involved in the oncogenic activity.
Besides all these technical issues, the cost of gene therapy is a bigger challenge in commercializing it.
It costs more than $ 100,000 per gene therapy.
Predicting the future of gene therapy is quite difficult because the results are uncertain and scattered. Notwithstanding it will be a dream that comes true for us if we succeed.
Many inherited diseases can be treated alongside life-threatening disorders like cancer.
Scientists are very close to treating monogenic disorders like cystic fibrosis and thalassemia. Furthermore, aggressive strategies for DMD and other related disorders suggest that there will be a lot of progress observed in the coming years.
However, gene therapy can be also be practiced incorrectly.
Gene therapy can be misused in enhancing athletic performance, increase longevity, stopping the aging process, and gaining more power (superhuman capability).
All these activities are unnatural and can unbalance the natural phenomenon, therefore, we have to use it from discretion.
Conclusion:
Inserting a transgene or replacing a faulty gene is a tedious phenomenon, which, requires, advanced instrumentations, precisions, and expertise.
All actions are subjected to total aseptic conditions, a minor contamination/infection with a virus can contaminate the entire cell line and the patients too.
Altering the embryo and changing the genetic composition is unethical and against natural laws. In broader prospects, the manifestation is through nature only, henceforth, the human rights wing and government both are on VETO for this.
Sources:
Scheller EL, Krebsbach PH. Gene therapy: design and prospects for craniofacial regeneration. J Dent Res. 2009;88(7):585-596. doi:10.1177/0022034509337480.
Gonçalves GAR, Paiva RMA. Gene therapy: advances, challenges and perspectives. Einstein (Sao Paulo). 2017;15(3):369-375. doi:10.1590/S1679-45082017RB4024
![Gene Therapy: Types, Vectors [Viral and Non-Viral], Process, Applications and Limitations](https://geneticeducation.co.in/wp-content/uploads/2019/06/new-baanner-.001-e1560763693164.jpeg)
can we get the pdf of this topic its very informative to discuss during our journel club
Sorry we don’t have that.
Nice Post! Thanks for sharing a great post.
Very informative! I wasn’t aware about these skin treatments. Now found something different and effective. Thanks for sharing this great article. https://tagband.co.uk/what-are-skin-tags-and-why-do-they-form/
This was very informative and helpful for my grade 12 project, Thank you for this.
Thank you sradha Kamath
great info